1.1: Introduction to Spectoscopy
- Page ID
- 189747
The structure of a compound has a big effect on its properties. But how do we know what that structure is?
The most useful methods of determining molecular structure involve the interaction of electromagnetic radiation, or light, with matter. Visible light, ultraviolet and infrared radiation, and even microwaves and radio waves interact with matter. They can each tell us different kinds of information about the materials they interact with.
How does light interact with matter?
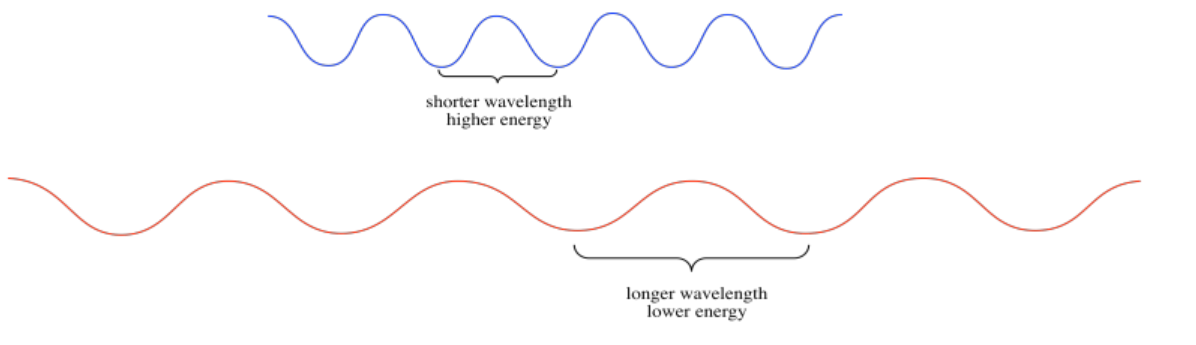
Light has wave properties, much like the waves you could see at the ocean shore. In physics, waves can be described in a number of different ways. Waves have amplitude: there are waves that rise very tall, and others that are low. Waves also have wavelength: they may have long wavelengths, with big distances from the peak of one wave to the peak of the one coming behind it. They may have short wavelengths, with one following very closely behind another. Wavelength gives rise to a complementary property, which is frequency. WHen waves are close together, you can see or hear them crashing to the shore very frequently. When they are farther apart, they seem to crash to the shore with a much lower frequency.
The different colors of light that we see have different wavelengths; blue light has a shorter wavelength than red light, for example. These different wavelengths of light have different amounts of energy. This idea is described in the Planck-Einstein relation:
\[E= h \nu \nonumber\]
(where E = energy, h = Planck's constant, n = frequency)
or
\[E = \frac{hc}{\lambda} \nonumber\]
(where c = speed of light, λ = wavelength)
This equation means:
- Higher frequencies of light are more energetic than lower frequency ones (when the number ν gets bigger, the number E also gets bigger).
- Higher frequencies correspond to shorter wavelengths (so when the length λ gets longer, E gets smaller).
There are a couple of important and surprising points about the interaction of photons with matter:
- Light is quantized; it travels in packages, called photons, and different photons have specific amounts of energy.
- Absorption of light by matter is also quantized; only specific packages or "quanta" can be absorbed by a specific material.
- Consequently, specific compounds absorb specific frequencies of light and don't absorb others.
When ultraviolet and visible light are absorbed, the energy from the light is transferred to an electron. The electron is excited to a higher energy level. Only certain energy levels are available in a material, and so the material can only absorb certain photons. That means:
- A photon with not enough energy to reach another energy level is not absorbed.
- A photon with too much energy to reach another energy level is not absorbed, either; the electron cannot absorb some of the energy from a photon and have a little left over for later.
- The wavelength or frequency of a photon that is absorbed by the electron corresponds to the amount of energy needed to reach another energy level.
The same sort of event can happen "backwards": an electron can lose energy by falling to a lower energy level. The lost energy can be given up by the electron as a photon of light. The wavelength or frequency of the photon corresponds to the difference between electron energy levels. This phenomenon, in which light is absorbed by a material and then given off again, is called "fluorescence".
There are many kinds of electromagnetic radiation.
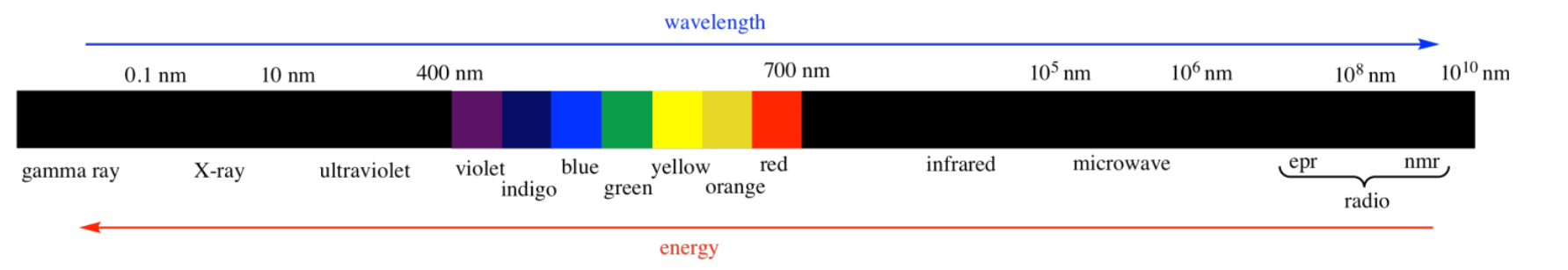
Many of these kinds of "light" can provide different kinds of information about structure. For example:
- UV-Visible spectroscopy tells us something about the electronic levels in a material. We will see more about this type of spectroscopy in another chapter. It is also discussed in a number of other places, in situations when it relates to molecular orbitals, to coordination compounds, and to photochemical reactions.
- X-rays can be used to construct an exact three-dimensional map of where the atoms lie in a crystalline material based upon how the x-rays scatter as they pass through the crystal. X-ray crystallography is a little bit too complicated for us, however.
- Radio waves interact with nuclear particles in a way that is similar to the absorption of UV light by electrons. However, this phenomenon only occurs in a strong magnetic field. The absorption of radio waves by the hydrogen nuclei in water molecules in human tissues is referred to as magnetic resonance imaging (MRI). The observation of nuclei in small molecules by a similar technique is referred to as nuclear magnetic resonance (NMR). NMR spectroscopy will be the subject of another chapter.
- Infrared light is absorbed by different bonds in a molecule. Infrared spectroscopy is the subject of another chapter.


