5.7: Optical Rotation
- Page ID
- 189638
- An equal mixture of two enantiomers is called a racemic mixture or racemate.
- If two enantiomers rotate plane-polarized light in opposite directions, a racemate will not rotate light at all. The effects of the two enantiomers will cancel out.
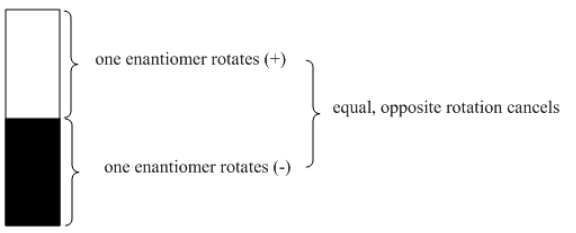
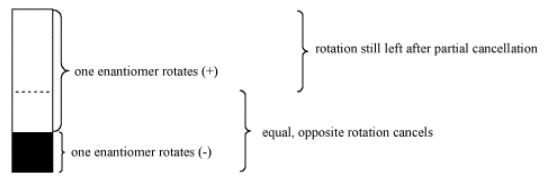
e "optical purity" is a comparison of the optical rotation of a pure sample of unknown stereochemistry versus the optical rotation of a sample of pure enantiomer. It is expressed as a percentage. If the sample only rotates plane-polarized light half as much as expected, the optical purity is 50%.
Optical purity also corresponds to "enantiomeric excess". If the unknown sample rotates light 50% as much as a sample of pure enantiomer, it must contain 50% enantiomeric excess; the other 50% is a racemic mixture. In other words, if the sample is 75% of one enantiomer and 25% of the other, 50% of the mixture will simply cancel out in terms of optical activity. The remaining 50% will still exert optical activity, but only half as much as if the sample were 100% of that enantiomer.
These relationships could be expressed in formulae:
\[\textrm{Optical purity (op)} = \frac{(\textrm{optical rotation of pure compound})}{(\textrm{optical rotation of pure enantiomer})} \times 100\% \nonumber\]
Enantiomeric excess (ee) = optical purity (that is, these numbers are always the same, although they represent different things)
\[\% \textrm{major enantiomer} = \textrm{enantiomeric excess} + \frac{100 - \textrm{enantiomeric excess}}{2} = 50 + \frac{\textrm{enantiomeric excess}}{2} \nonumber\]
\[\% \textrm{minor enantiomer} = 100 - \% \: \textrm{major enantiomer} \nonumber\]
Exercise \(\PageIndex{1}\)
The (+) enantiomer of compound A has an optical rotation of 75o. If a sample containing only compound A has an optical rotation of 50o, what is the composition of the sample?
- Answer
-
A pure sample of A would have \([\alpha] = 75^{o}\)
Optical purity or enatiomeric excess \( = \frac{50}{75} = 66 \%\)
% major enantiomer \(= 66 + \frac{34}{2} = 83 \% \)
% minor enantiomer \( = 100 - 83 = 17 \% \)
Exercise \(\PageIndex{2}\)
The (+) enantiomer of compound B has an optical rotation of 50o. If a sample containing only B contains 10% of the (+) enantiomer and 90% of the (-) enantiomer, what is the optical rotation value?
- Answer
-
\[ \% major = 90 \% \nonumber\]
\[ \% minor = 10 \% \nonumber\]
Optical purity or enantiomeric excess \( = \frac{X}{-50} = 90 -10 - 80 \% \)
Solve for X.
\[X = -40 ^{\circ} \nonumber\]
Exercise \(\PageIndex{3}\)
The (-) enantiomer of compound C has an optical rotation of -60. A sample of compound C is shown by chiral gas chromatography to contain only (-) C and no (+) C, but NMR analysis suggests the sample is about 50% ethyl acetate by weight. Predict the measured optical rotation of a 1 g/mL solution in dichloromethane, measured in a 1 dm cell.
Exercise \(\PageIndex{4}\)
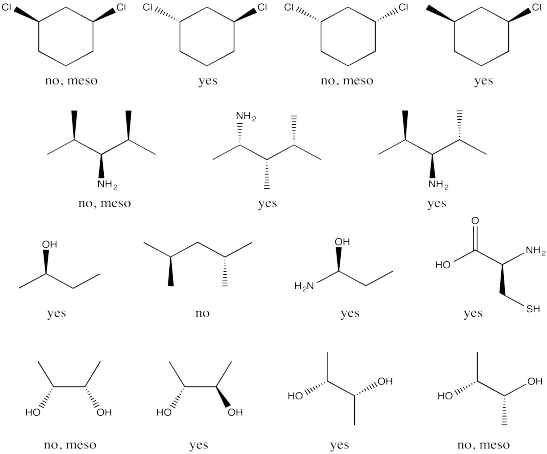
Which of the following compounds are optically active?
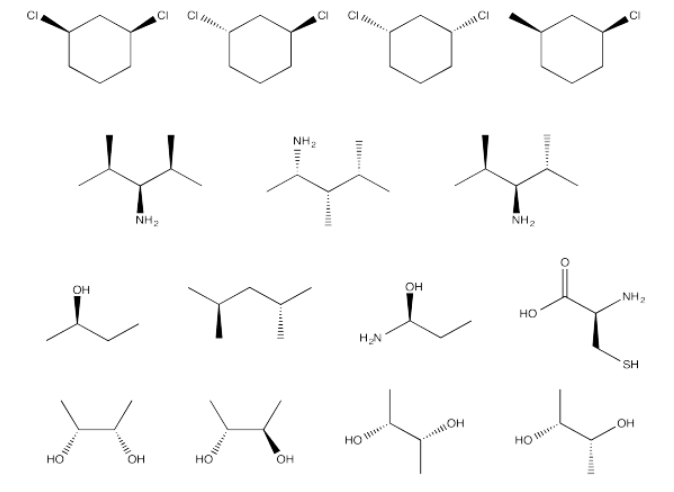
- Answer
-