4.16: Solutions to Selected Problems
- Page ID
- 191199
Exercise 4.1.1:
Exercise 4.1.2
Exercise 4.2.1:

Exercise 4.2.2
Exercise 4.3.1
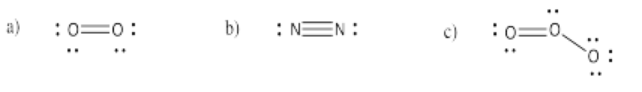
Exercise 4.3.2:
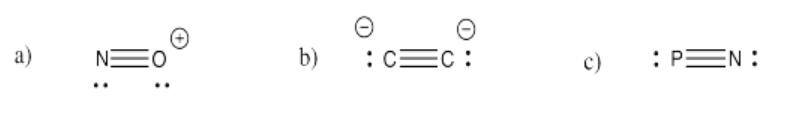
Exercise 4.3.3:
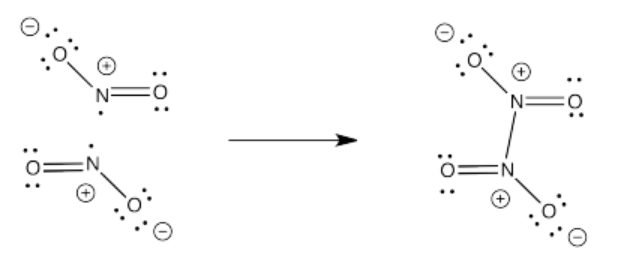
Exercise 4.4.1:
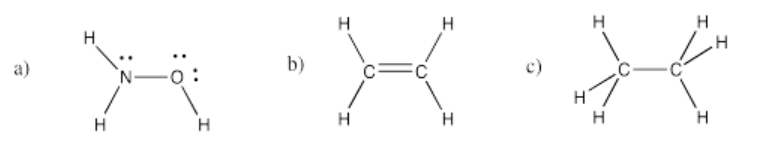
Exercise 4.4.2:
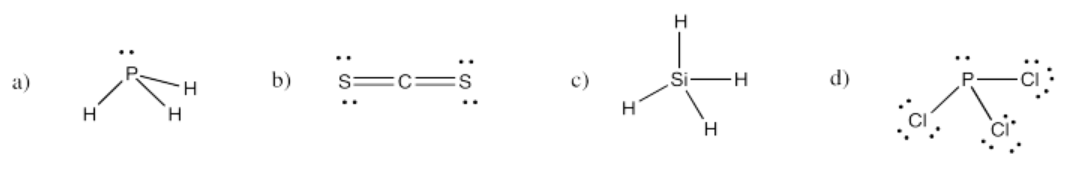
Exercise 4.4.3:
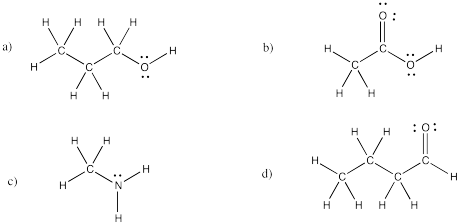
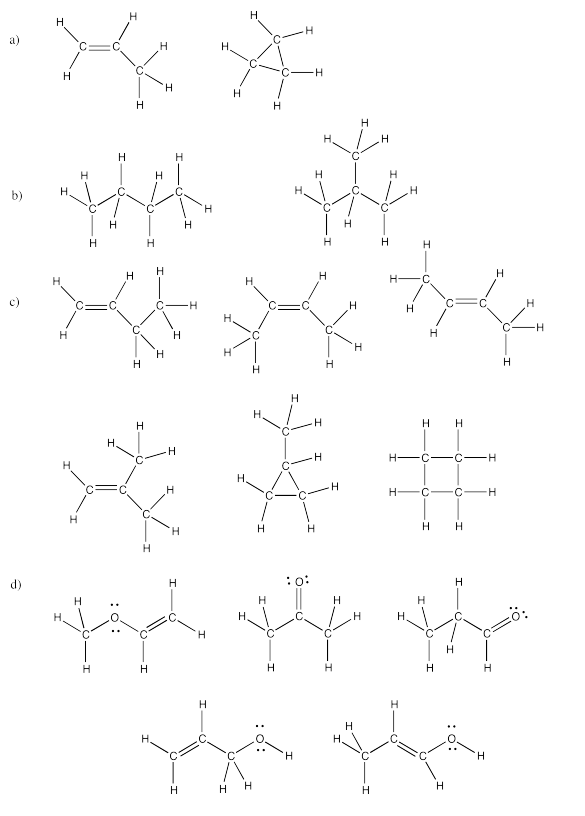

Exercise 4.4.5
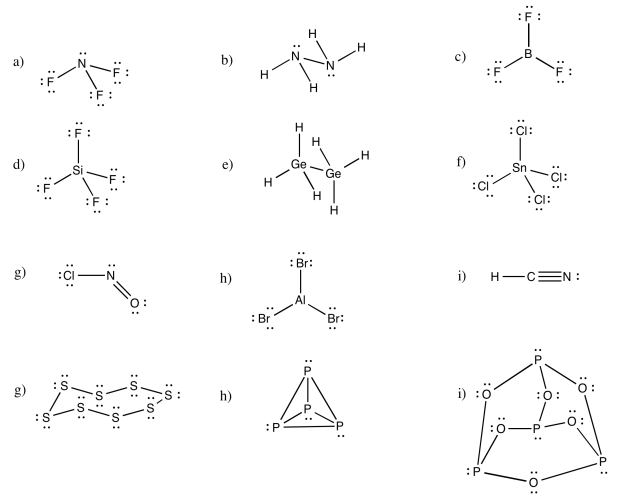
Exercise 4.5.1:
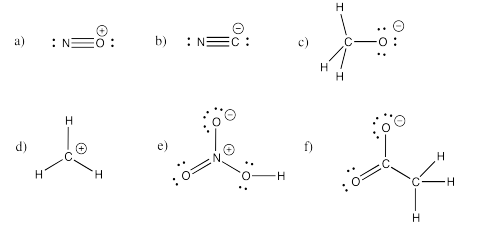
Exercise 4.5.2:
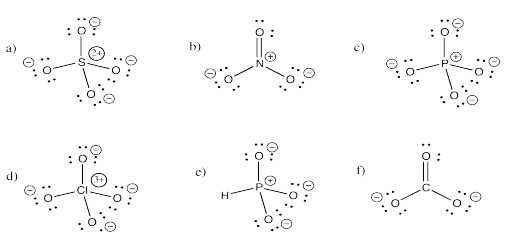
Exercise 4.5.3:
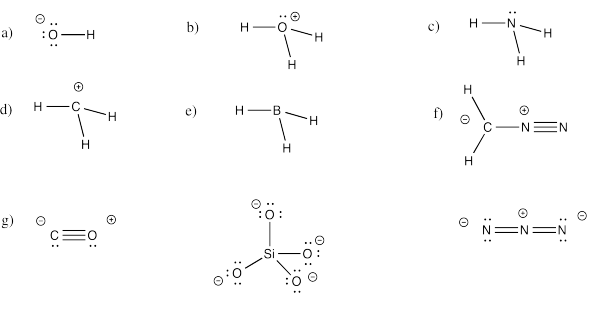
Exercise 4.5.4:
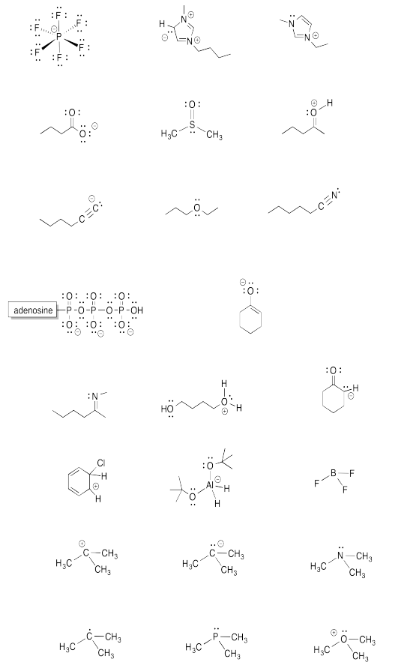
Exercise 4.6.1:
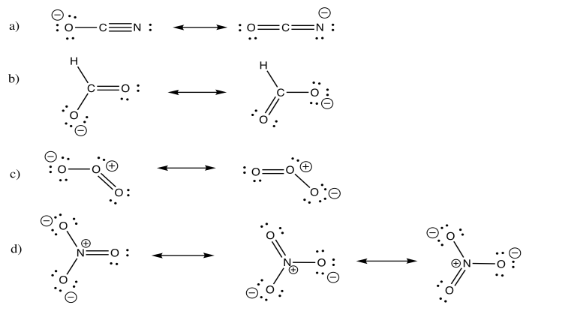
Exercise 4.6.2:
Exercise 4.6.3:
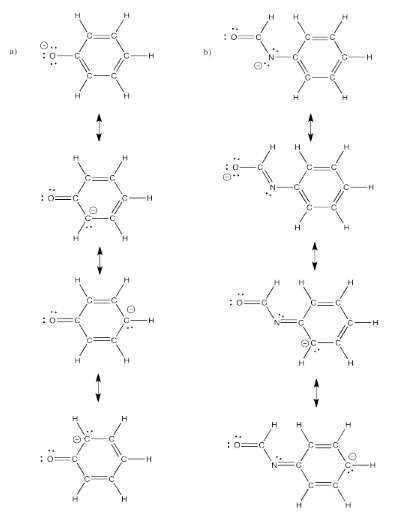
Exercise 4.6.4:
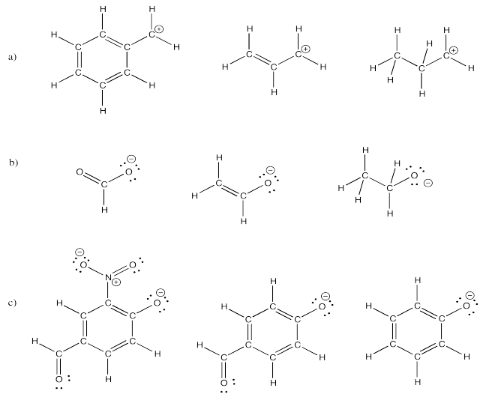

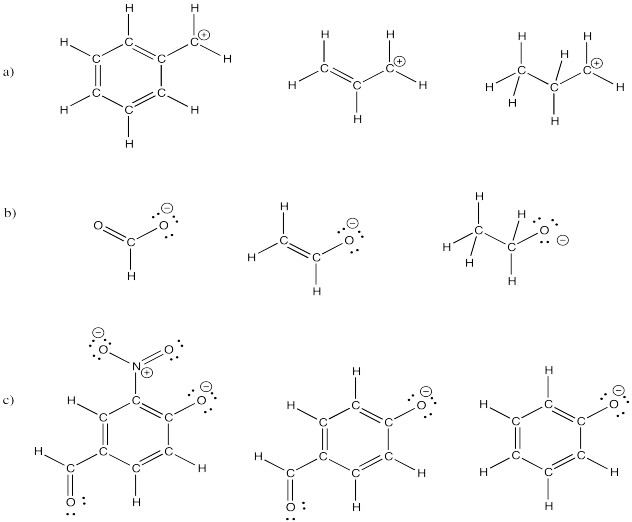
Exercise 4.6.5:
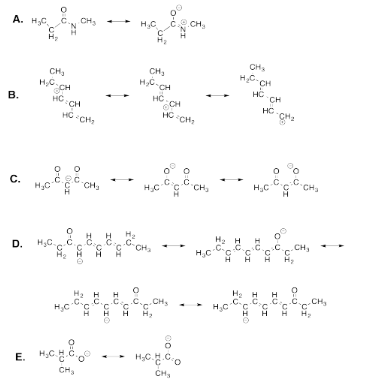
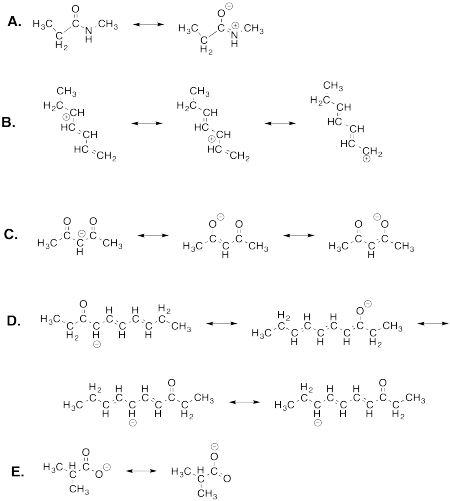
Exercise 4.7.1:
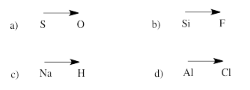
Exercise 4.7.2:
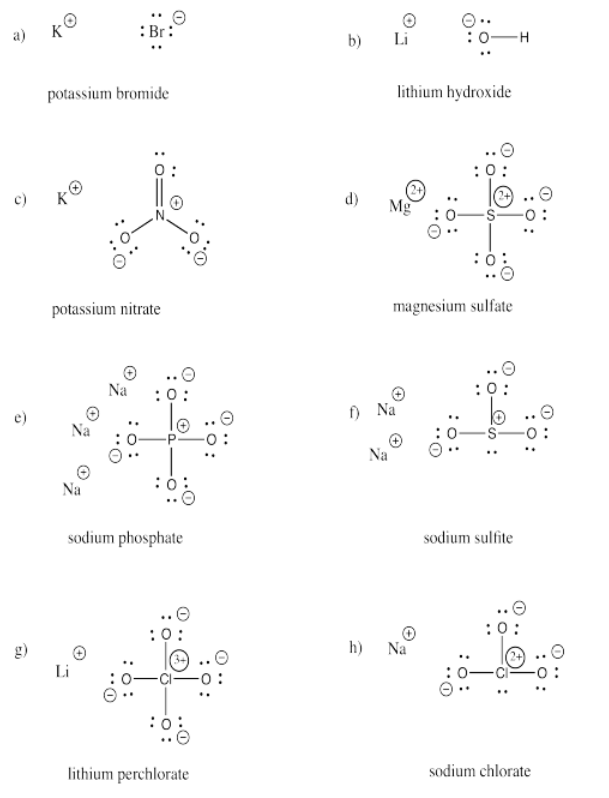
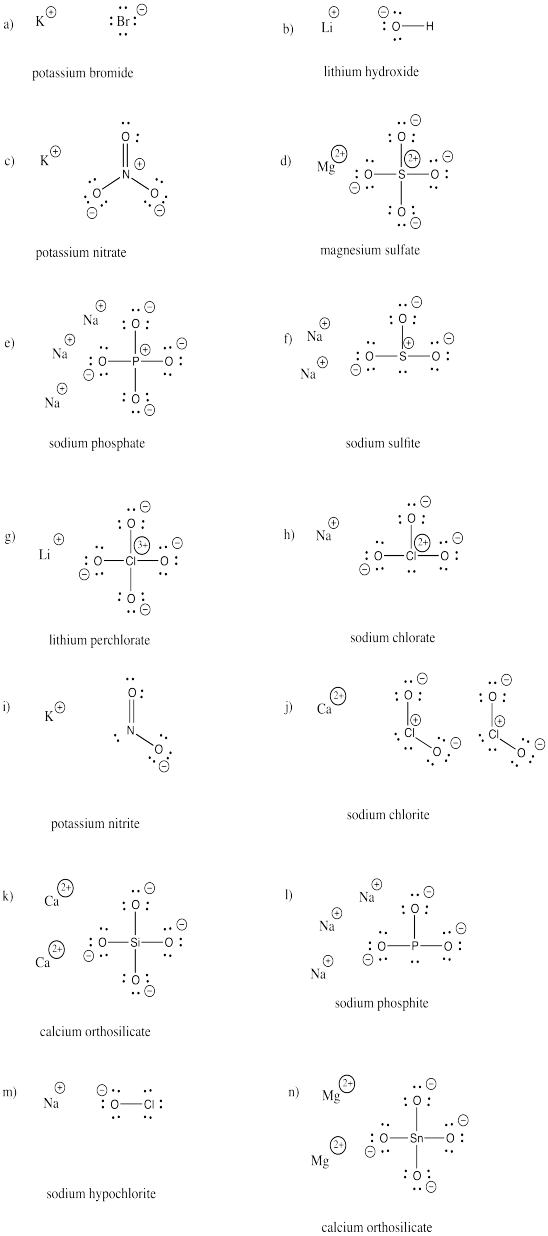
Exercise 4.7.3:
Exercise 4.7.4:
a) bromide b) oxide c) fluoride d) carbonate e) nitrate f) nitrite
g) sulfide h) sulfate i) sulfite j) persulfate k) carbide l) nitride m) arsenide
n) phosphate o) phosphite p) iodide q) iodate r) periodate
Exercise 4.8.1:
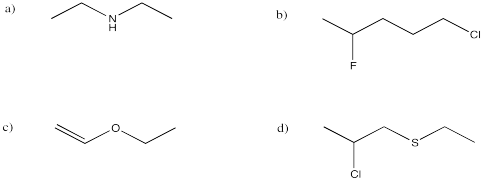
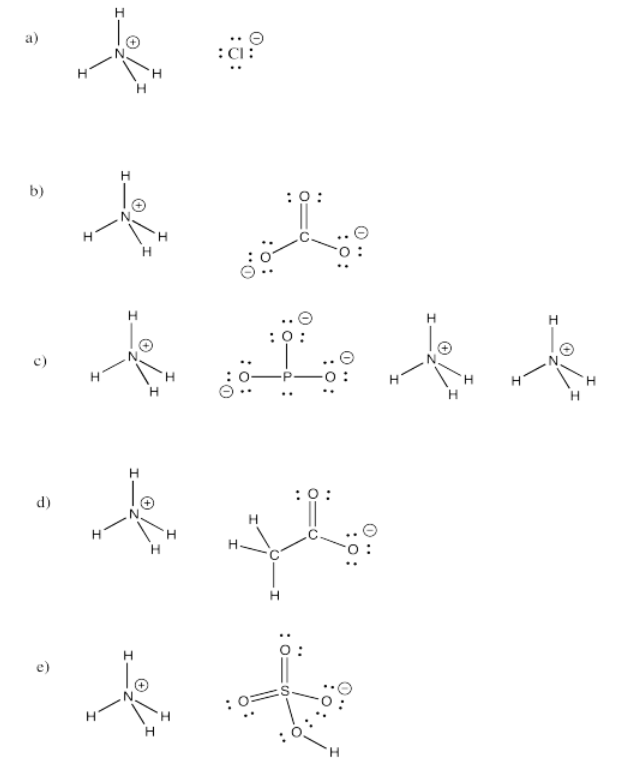
Exercise 4.8.2:
a) (CH3)2CHCH2CH2CN b) CH3CH2CH(OH)CH3
c) (CH3)2CHCOCH2CH3 d) CH3CH2CONHCH2CH3
Exercise 4.8.3:
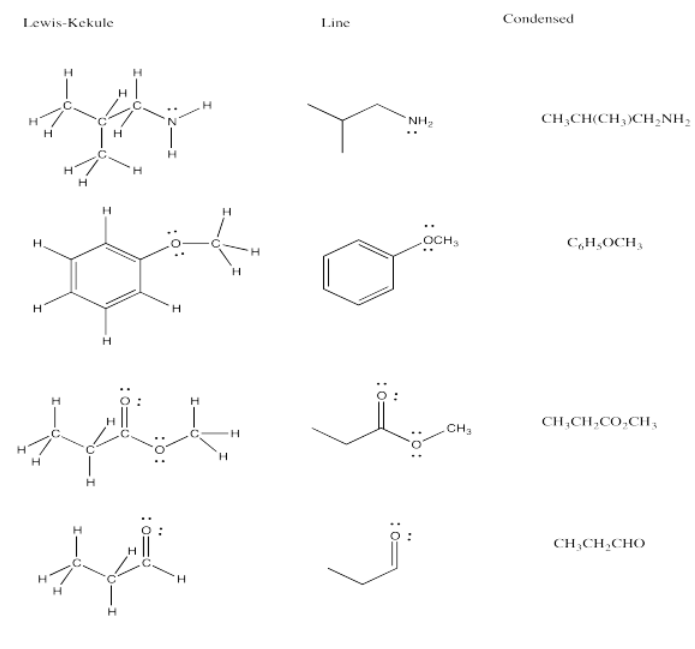
Exercise 4.8.4:
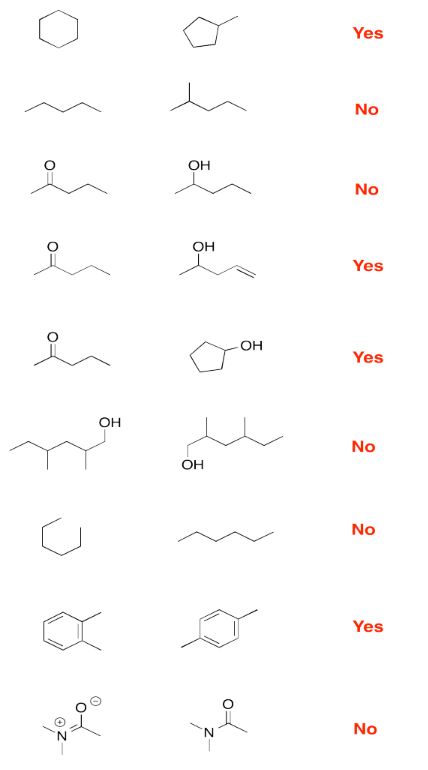
Exercise 4.9.1:
Exercise 4.9.2:
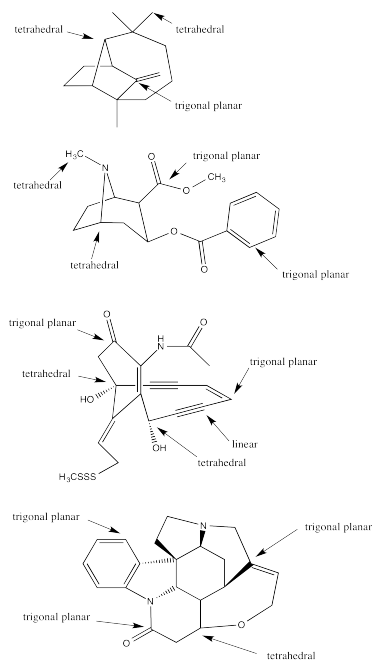
Exercise 4.10.1:
a) octahedral.
b)
c)
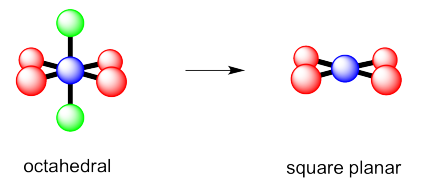
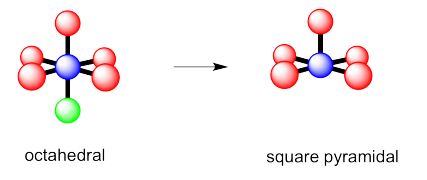
Exercise 4.10.2:
- trigonal bipyramidal
- This time there could be two different answers.
If the lone pair occupies one of the axial positions, it would be pretty close to three other atoms.
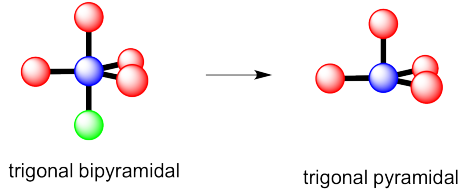
If the lone pairs occupies one of the equatorial positions, it would be pretty close to only two other atoms. The other equatorial atoms are pretty far away.
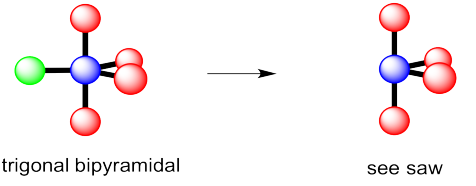
The rule is that the lone pair goes in the less crowded position, so this molecule would be see-saw shaped.
c) Again, there are two possible geometries. One of them would be trigonal planar, a pretty common geometry.
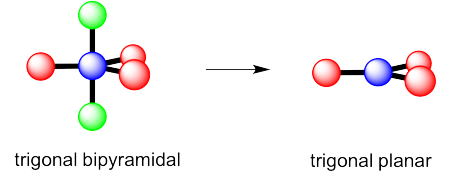
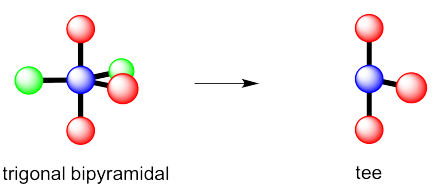
Exercise 4.10.3:
Exercise 4.10.4
a) bent b) pyramidal at O, although tetrahedral at C c) pyramidal
d) see-saw e) tee f) trigonal bipyramidal
g) octahedral h) square pyramidal i) square planar
Exercise 4.10.5:
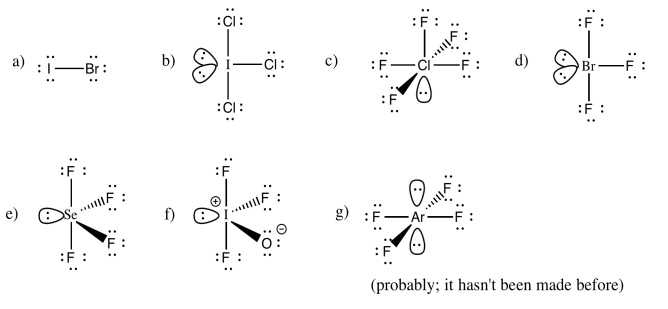
Exercise 4.10.6:
You may be able to imagine some other possibilites for this number of neighbors, but IF7 adopts a pentagonal bipyramid shape.
Exercise 4.11.1:
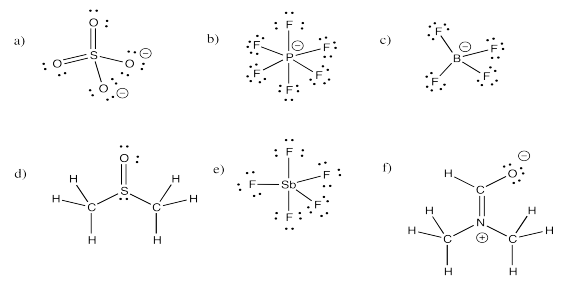
Exercise 4.11.2:
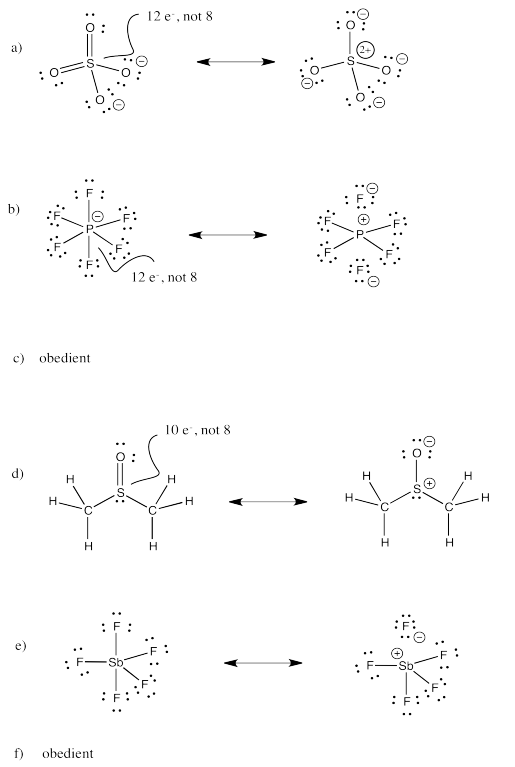
Exercise 4.11.3:
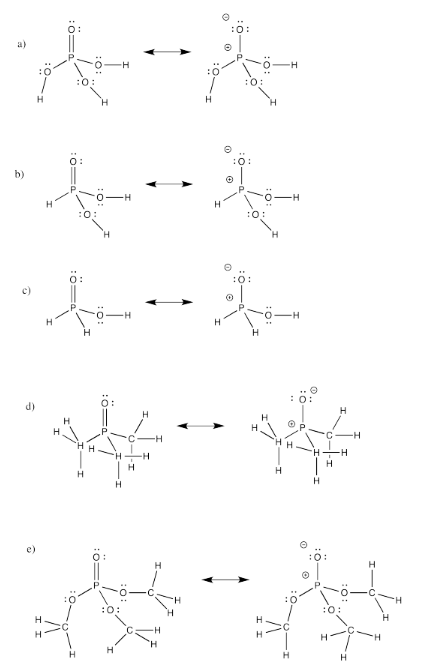
Exercise 4.12.2:
a) propane b) pentane c) hexane
Exercise 4.12.3:
a) 3-methylhexane b) 2,2-dimethylpentane c) 2,3-dimethylbutane
d) 2,2,3,3-tetramethylpentane e) 3,5-dimethylheptane f) 4-ethyl-3,6-dimethyloctane
Exercise 4.12.4
a) cyclopentane b) cyclohexane c) cyclooctane
d) methylcyclobutane e) 1,1,3-trimethylcyclopentane f) 1,3-dimethylcycloheptane
Exercise 4.12.5:
a) 1-hexene b) 2-methyl-2-pentene c) 1-methylcyclohexene d) 2,4,6-trimethyl-2-heptene
Exercise 4.12.6:
a) cyclopentene b) 1,1-dimethylcyclohexane c) 3-hexyne
d) 4-methylcyclohexene e) 1-hexyne
Exercise 4.12.7:
a) tetrahedral b) trigonal planar c) linear
Exercise 4.12.8:
a) methylbenzene b) propylbenzene c) 1,2-dimethylbenzene or o-dimethylbenzene (also o-xylene)
d) 1,3-dimethylbenzene or m-dimethylbenzene (also m-xylene) e) 1,4-diethylbenzene or p-diethylbenzene
f) 2-ethyl-1,4-dimethylbenzene
Exercise 4.12.9:
a) 2,2-dimethylhexanal b) 2-methylcyclopentanone
c) 3-nonanone d) 2,4-dimethyl-2-hexenal
Exercise 4.12.10:
a) butyl propanoate b) N,N-diethylbutanamide
c) 6-methylheptanoic acid d) 4-pentenoic acid
Exercise 4.12.11:
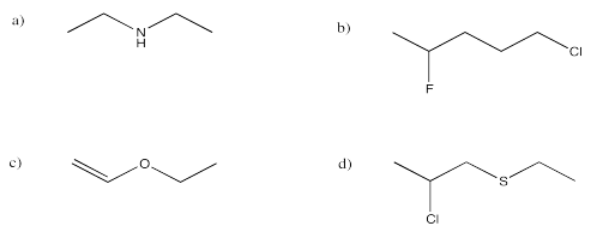
a) 1-chloro-2-methylcyclohexane b) cyclooctanol c) ethyl cyclopentyl ether
d) N-propylcyclohexylamine e), 5,5-dimethylheptan-2-ol f) 3-bromo-4,4-dimethyloctane
g) dibutylamine i) methyl phenyl ether (or anisole) j) ethane thiol k) diethyl thioether
l) triethylphosphine m) butanenitrile n) nitromethane
Note that sometimes a number is located directly in front of the suffix for the group to which it refers.
Exercise 4.12.12
- benzene (or aromatic), ketone and ether
- bromide, amine and aldehyde
- alcohol, thiol and ester
- thioether, amide and alkene
- alkyne, alcohol and carboxylic acid
Exercise 4.12.13
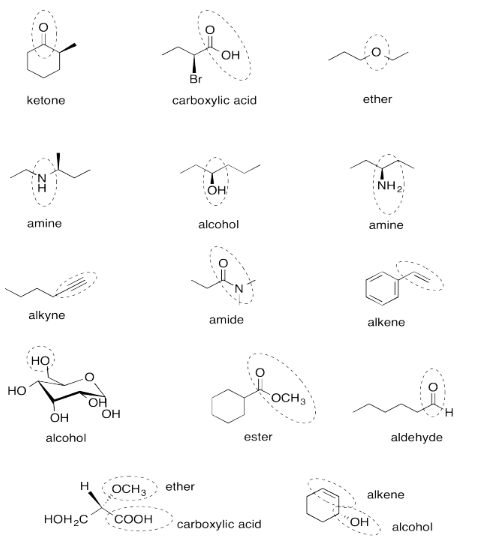
Exercise 4.12.14
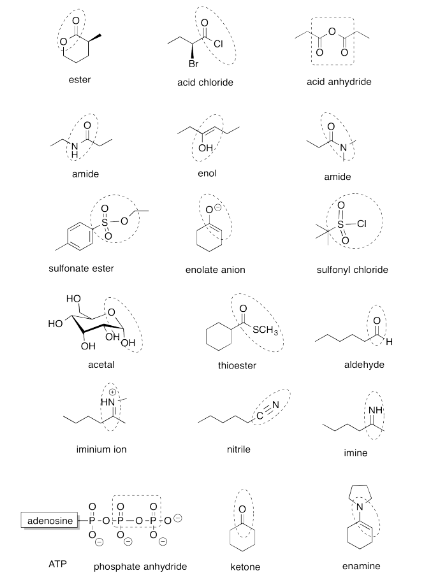
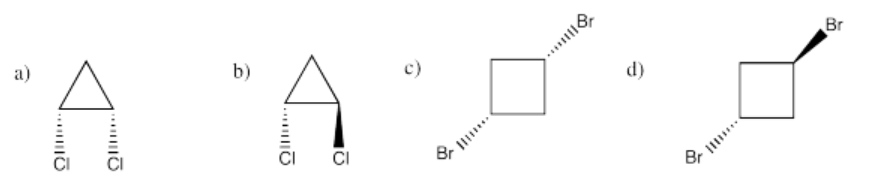
Exercise 4.13.1:
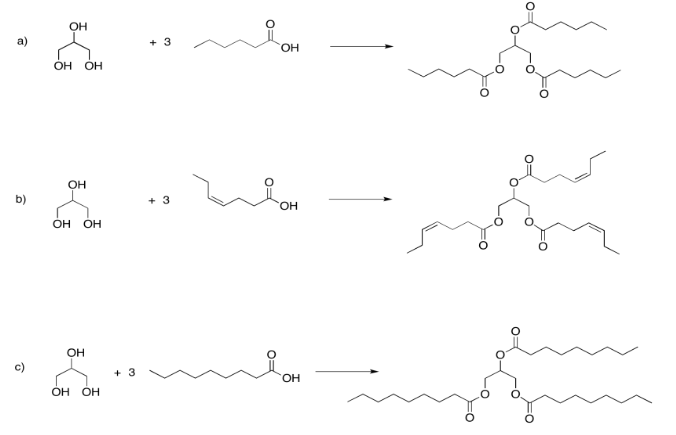
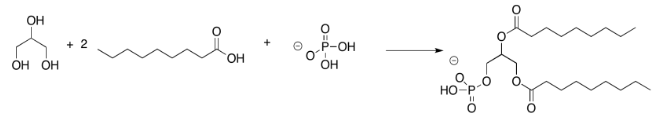
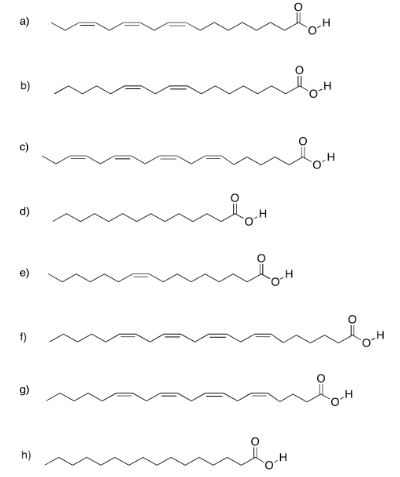
Exercise 4.13.4:
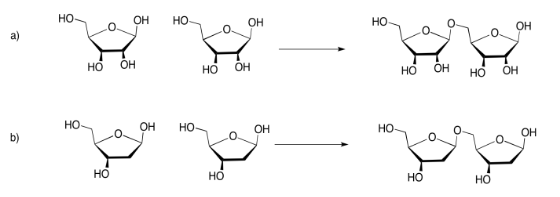
Exercise 4.13.5:
If you don't know what the wedged and dashed lines in the drawing mean, don't worry about it. They just represent different orientations in space. You will learn about these representations in a later topic called "stereochemistry".
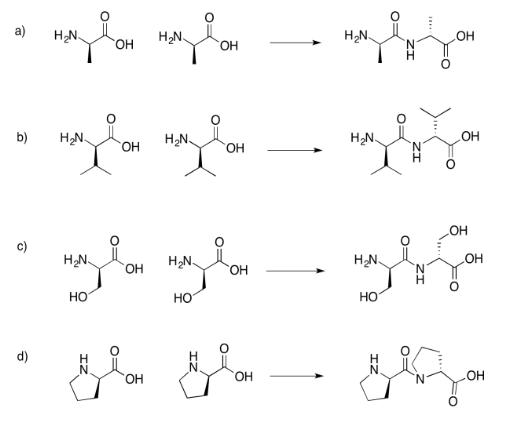
Exercise 4.13.6:
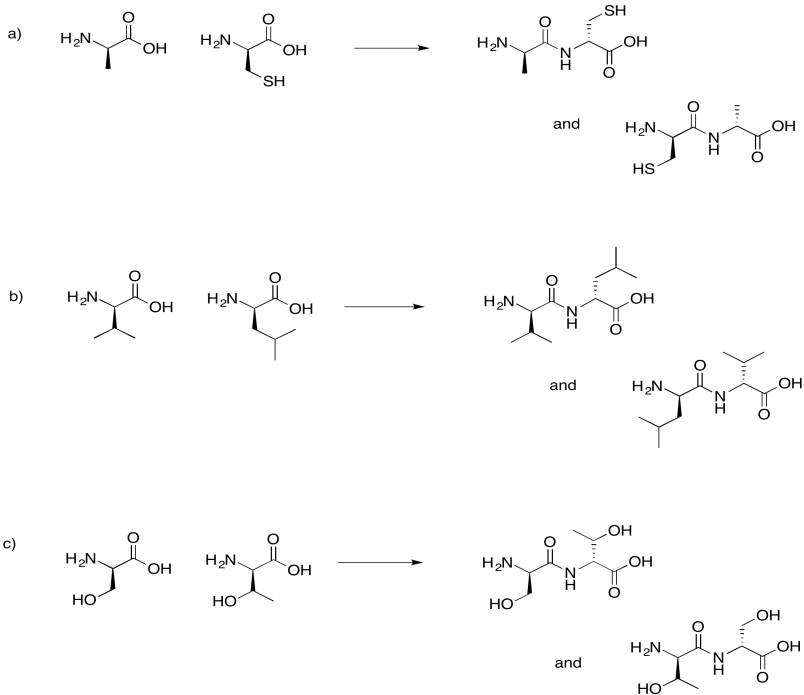
Exercise 4.13.7:
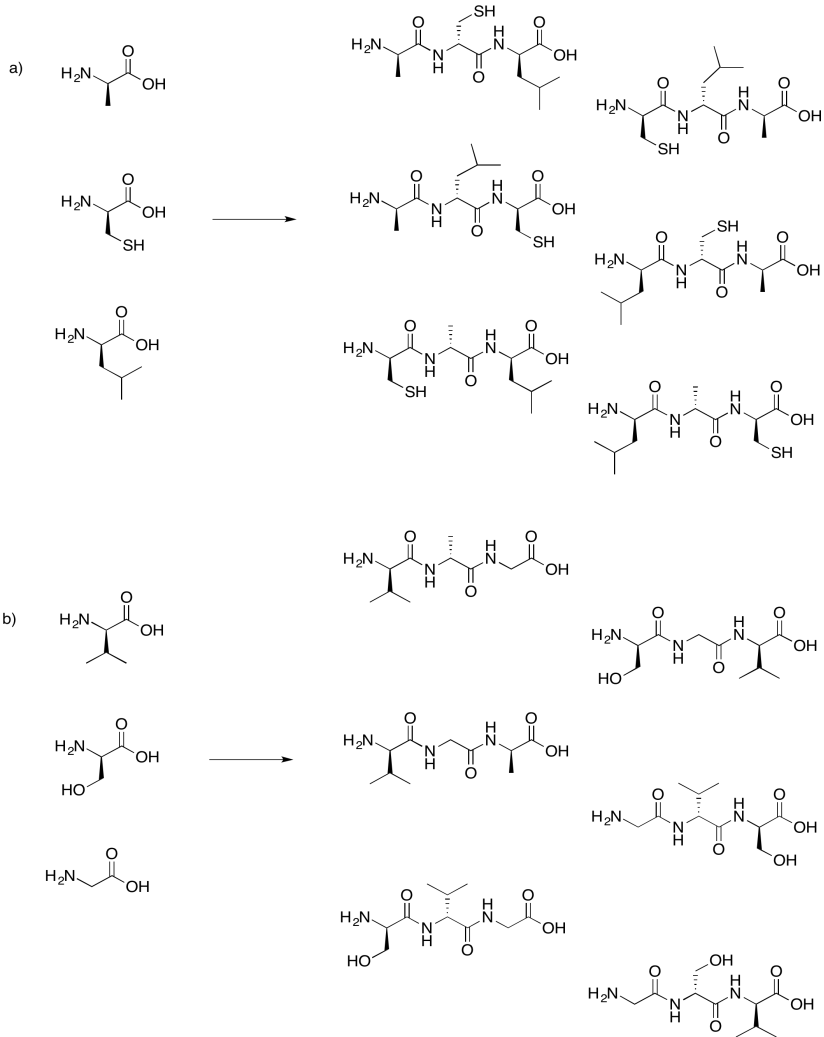
Exercise 4.13.8:

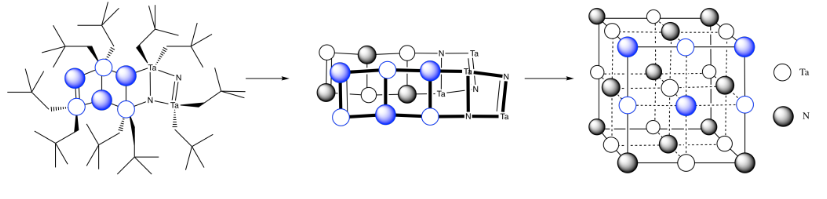
Exercise 4.15.1:
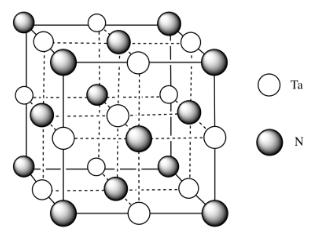
- N3- would get to a noble gas configuration.
- Ta3+ would balance the charge in TaN.
c)
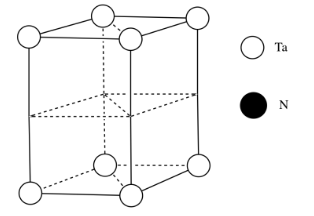
d)
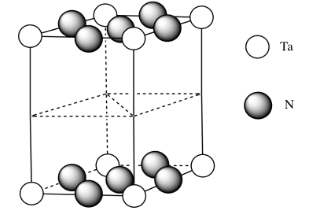
e) \( \# Ta = (\frac{1}{6})(\frac{1}{2})(4) \) for the acute corners and \((\frac{1}{3})(\frac{1}{2})(4)\) for the obtuse corners = \(\frac{4}{12} + \frac{4}{6} = \frac{4}{12} + \frac{8}{12} =1\)
(note that it's the same outcome as the corners of a cube)
f) \( \# N = \(\frac{1}{4})(8)\) for the edges and \((\frac{1}{2})(2)\) for the faces = \( 2 + 1 =3\)
g) Need 2 more Ta.
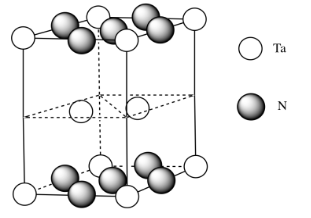
h) Each tantalum has three nitrogens above and three below it. It's almost octahedral, but the top layer of nitrogens is lined up above the bottom layer rather than being twisted 120 degrees to form an octahedron. The geometry is a trigonal prism.
i)
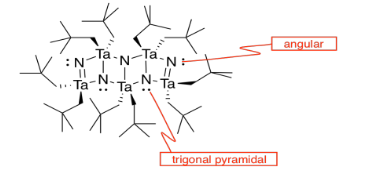
j)
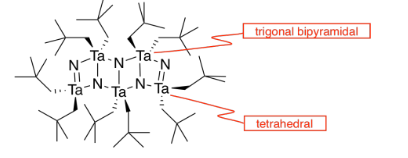
k)
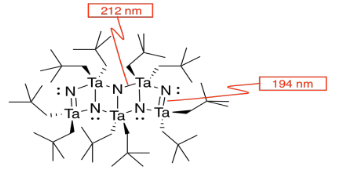
l) The double bonds hold the atoms more closely together than the single bonds.
m) You can imagine the molecules stacking together to make a cubic array of TaN.