Dipole-dipole Forces
- Page ID
- 53536
Skills to Develop
- Define and illustrate dipole-dipole forces
Dipole-dipole forces are probably the simplest to understand. You probably already know that in an ionic solid like NaCl, the solid is held together by Coulomb attractions between the oppositely-charges ions. The Na+ and Cl- ions alternate so the Coulomb forces are attractive. Dipole-dipole forces work the same way, except that the charges are smaller. A good example is HF (this is also an example of a special type of dipole-dipole force called a hydrogen bonding). In HF, the bond is a very polar covalent bond. That means there is a partial negative (δ-) charge on F and partial positive (δ+) charge on H, and the molecule has a permanent dipole (the electrons always spend more time on F). In the liquid or solid HF, the molecules arrange themselves so that the δ- and δ+ are close together. These partial charges attract each other, and this attraction is what we call dipole-dipole forces. Any molecule with a permanent dipole has dipole-dipole forces that hold the molecules next to each other as a solid or liquid.
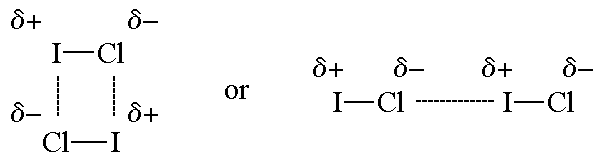
Outside Links
- Khan Academy: States of Matter (19 min)
- Khan Academy: Van der Waals Forces (12 min)
- CrashCourse Chemistry: Liquids (11 min)
Contributors and Attributions
Emily V Eames (City College of San Francisco)

