21.1: Prelude to Spectroscopy
- Page ID
- 50594
In the following sections we are going to study the way in which matter can both absorb energy and emit it in the form of electromagnetic radiation such as light. The pattern in which matter absorbs or emits radiation is called its spectrum. In the past, and still to this day, studies of the spectrum of a substance have furnished important clues to the structure of matter. At the same time, the spectrum of a substance is often a very useful way of characterizing and hence identifying and analyzing that substance.
Many of the properties of electromagnetic radiation can be explained if light is thought of as periodically varying electric and magnetic fields (electromagnetic waves). Such waves can be characterized by their frequency v or their wavelength λ, and their speed of propagation is always λv = c = 2.998 × 108 m s–1. Some properties of light are more easily explained in terms of particles called photons. The energy of a photon is given by E = hv, where h = 6.626 × 10–34 J s and is called Planck’s constant.
When any element is heated to a high temperature or excited in a discharge tube, it gives a line spectrum. Niels Bohr was able to predict the wavelengths of the lines in the spectrum of hydrogen by means of a theory which assigned the single electron to specific energy levels and hence to orbits of specific radius. Absorption of an appropriate quantity of energy can raise the hydrogen atom from a lower to a higher energy level, while emission of electromagnetic radiation corresponds to a change from a higher to a lower energy level. Although Bohr’s theory is quantitatively accurate only for hydrogen, his idea of energy levels is useful for all other atoms and even for molecules.
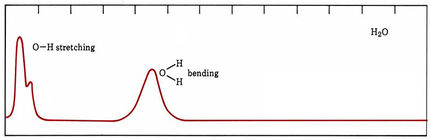
In the case of molecules, energy levels arise because of different speeds and kinds of molecular vibrations and rotations as well as because electrons are moved farther from or closer to positively charged nuclei. In organic compounds some groups of atoms vibrate at much the same frequency no matter what molecule they are in. The energy levels of such vibrations usually differ by roughly the energies of infrared photons, and many organic functional groups can be identified by the characteristic frequencies at which they absorb infrared radiation. When molecules absorb visible or ultraviolet light, band spectra occur. Some of the energy of each absorbed photon goes to excite an electron, but varying amounts also increase vibrational and rotational energies. Thus photons are absorbed over a broad range of frequencies and wavelengths.
The most convenient theory by which the electronic energies of molecules can be predicted is the molecular-orbital theory. It assumes that electrons in a molecule occupy orbitals which are not confined to a single atom but rather extend over the entire molecule. Bonding molecular orbitals involve constructive interference between two electron waves, while antibonding molecular orbitals involve destructive interference. An electron occupying an antibonding MO is higher in energy than it would be if the atoms were not bonded together, and so antibonding electrons cancel the effect of bonding electrons. This explains why molecules such as He2 or Ne2 do not form.
Molecular-orbital theory is especially useful in dealing with molecules for which resonance structures must be drawn. Because molecular orbitals can be delocalized over several atoms, there is no need for several resonance structures in the case of molecules like O3 and C6H6. The greater the extent of electron delocalization, the smaller the separation between molecular energy levels and the longer the wavelength at which absorption of ultraviolet or visible light can occur. Thus compounds containing long chains of alternating single and double bonds or having several benzene rings connected together often absorb visible light and are colored.


