20.2: The Elements of Life
- Page ID
- 49599
Hardly a year goes by without the appearance in the media of the old cliché that the average human body is worth about $3.57 (perhaps a little more, allowing for inflation). Such figures are based on the elemental composition of the human body reported in the table.
|
Element
|
Weight %
|
Atom %
|
Period
|
|---|---|---|---|
|
H
|
10.2
|
63.5
|
I
|
|
O
|
66.0
|
25.6
|
II
|
|
C
|
17.5
|
9.1
|
II
|
|
N
|
2.4
|
1.06
|
II
|
|
Subtotal
|
96.1
|
99.3
|
|
|
Ca
|
1.6
|
0.25
|
IV
|
|
P
|
0.9
|
0.18
|
III
|
|
Na
|
0.3
|
0.07
|
III
|
|
K
|
0.4
|
0.06
|
IV
|
|
Cl
|
0.3
|
0.05
|
III
|
|
S
|
0.2
|
0.04
|
III
|
|
Mg
|
0.05
|
0.01
|
III
|
|
Subtotal
|
99.85
|
99.92
|
|
|
Fe
|
0.005
|
0.0006
|
IV
|
|
Zn
|
0.002
|
0.0002
|
IV
|
|
Cu
|
0.0004
|
0.000 06
|
IV
|
|
Mn
|
0.000 05
|
0.000 006
|
IV
|
Considering just the market value of the elements, one obtains a ridiculously low price. What has been ignored, of course, is how the atoms of those elements are put together. The raw materials for a fine watch are not worth much either-what we pay for is mainly the skill and intelligence with which they are combined.
Before considering how the elements from which we all are made have been combined, however, it is worth thinking a little about why those particular elements in the table are involved. This is especially important because the same elements in very similar ratios are found in nearly all living systems. As shown in the biological periodic table (Figure \(\PageIndex{1}\) ) a great many other elements are simply not involved in the chemistry of life-at least not to the extent that the necessity of their presence can be demonstrated experimentally.
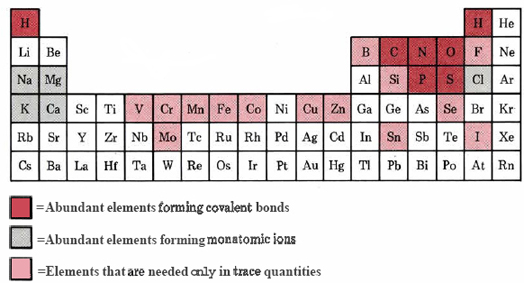
More than 99 percent of the atoms in the human body, or any other organism for that matter, are H, O, N, and C. This does not appear to be mere happenstance. The indications are that life as we know it could not be based on any other four elements. These elements have the lightest and smallest atoms which can form one, two, three, and four covalent bonds, respectively. Because of their small radii these atoms can approach very closely and bond very strongly to each other. The most important of these four elements is undoubtedly carbon. Carbon atoms have a special capacity for binding with each other to form long chains and rings. This capacity allows carbon to form a very large number of stable compounds whose molecular structures are different but nevertheless closely related.
No other element has this capacity. Perhaps the closest contender is silicon. Although a number of compounds containing Si—Si bonds such as
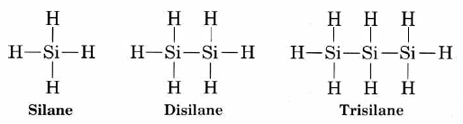
are known, none of these compounds are very stable. All are readily converted to compounds containing Si—O bonds. The bond enthalpy of the bond (368 kJ mol–1) is much larger than that of the Si—Si bond (176 kJ mol–1) and also larger than that of the Si—H bond (318 kJ mol–1). The production of Si—O bonds is thus exothermic and thermodynamically favorable. Another factor is the rapid rate at which these chemical reactions can occur. Silicon is capable of expanding its valence shell through the use of d orbitals to allow more than four bonds. This enables it to form an activated complex in which both the bond being made and the bond being broken feature, but which requires very little activation energy. Equivalent reactions involving carbon require very much higher activation energies and usually proceed so slowly as to be imperceptible at room temperature, even when thermodynamically permitted.
Carbon is not the only element with unique properties in biological molecules. Hydrogen is also special and plays two important roles. You will recall that alkanes are chemically unreactive. We can attribute this to the large C—H bond enthalpy of 413 kJ mol–1. Only fluorine forms a stronger bond than this with carbon. It is thus quite difficult to replace a hydrogen atom attached to a carbon atom with a more stable alternative. When an organic compound reacts chemically, it is almost always a functional group which undergoes a change. The presence of C—H bonds renders a large proportion of the carbon chain unreactive and restricts reaction almost entirely to those sites which include an atom other than carbon or hydrogen.
In biological molecules these other atoms are almost invariably oxygen and nitrogen, and this is no accident either. Oxygen and nitrogen are the two most electronegative elements which have a valence greater than 1. They are able to form bonds both to carbon on the one hand and to hydrogen on the other. In groups like
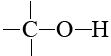 and
and 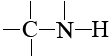
hydrogen atoms fulfill their second important role in biological molecules—forming hydrogen bonds between different molecules or between different parts of the same molecule.
This ability of different functional groups on a carbon chain to hydrogen bond with each other is a particularly important aspect of biological molecules. You will recall from figure 2 in the section on molecular equilibrium that a molecule containing a chain of carbon atoms is capable of a very large number of conformations due to free rotation around the single bonds. Since many biological molecules contain very long chains, they are capable of adopting an almost infinite number of shapes. In practice only a few of these shapes are useful, and the molecules can be "frozen" into such a useful conformation through hydrogen-bonding between various segments of the chain. A very good example of this is the enzyme trypsin. If the amino acid chain in this molecule were not held in the particular conformation shown in the figure by means of hydrogen bonds between adjacent parts of the chain, the various segments making up the active site would no longer be grouped together and the molecule would be unable to function as a catalyst.


