8.6.1: Astronomy- Titan
- Page ID
- 50820
The simplest hydrocarbons are alkanes, compounds comprised of only carbon and hydrogen linked together by single bonds. Each carbon atom has tetrahedral geometry around it, with angles of 109.5°. Simple diagrams and three-dimensional jmol structures of three alkanes are shown below.
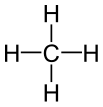 Methane |
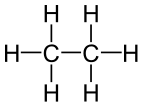 Ethane Ethane |
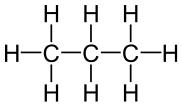 Propane |
|
Figure \(\PageIndex{1}\) Ball-and-stick model for the first three members of the alkane family: (a) methane, CH4; (b) ethane, C2H6; (c) propane, C3H8. |
Alkanes are very nonpolar due to their symmetry and general lack of dipoles between carbon and hydrogen atoms.
By observing the general size and nonpolarity of the molecules shown above, what can you infer about the intermolecular forces between them? Relate the presence of these forces to expected melting and boiling points of these alkanes compared to each other and to a more polar molecule, such as water.
Answer
The alkanes shown have almost no net dipole and are relatively small molecules. Therefore, the only intermolecular forces involved are London forces. Because propane is larger there will be more London attraction forces holding propane molecules together compared to ethane and methane, so propane will have higher melting and boiling points. However, the forces are still quite weak, and we would expect these alkanes to have lower melting and boiling points than a polar molecule such as water.
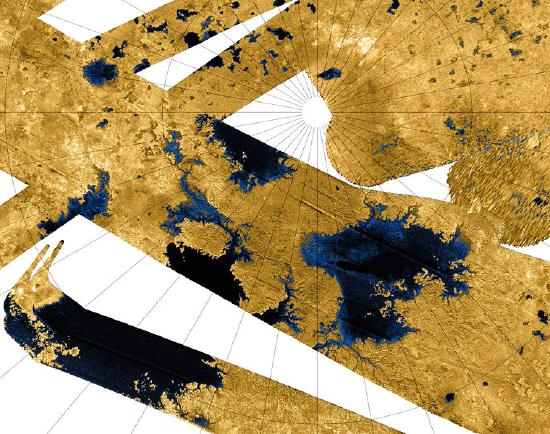
The intermolecular forces in each molecule are weak enough to the point where methane, ethane, and propane are normally found as gases at room temperature. These hydrocarbons exist in concentrated areas of space, most notably on the Saturnian moon Titan. Titan is unique in that in has a very dense atmosphere rich in hydrocarbons and lakes of simple alkanes[1]. Bodies of methane and ethane play almost the same role on Titan as liquid does on Earth. The heavier propane, used by humans as a fossil fuel, is present in excess of 700 million Earth barrels[2]. Scientists are currently attempting to figure out just what produces these hydrocarbons on Titan, but for now, let's explore the geometry and nomenclature of these three molecules further.
Methane, Ethane, and Propane
Ball-and-stick models of alkane molecules which contain up to three carbon atoms are shown in Figure 1. Methane (a) has four C—H bonds arranged tetrahedrally around a single carbon atom. Ethane (b) has a slightly more complicated structure—each carbon is still surrounded tetrahedrally by four bonds, but only three are C―H bonds, while the fourth is a C—C bond. Ethane may be thought of as two methyl groups connected by a single C—C bond. (The methyl group, CH3—, has the same structure as methane except that one hydrogen has been removed.) Thus the formula for ethane is CH3CH3 or C2H6.
The third member of the alkane family is propane. As can be seen in part c, the three carbon atoms are in a chain. The two methyl groups at the ends of the chain are linked together by a methylene group, —CH2—. The formula is CH3CH2CH3 or C3H8. Again the tetrahedral arrangement of C—H or C—C bonds around each carbon atom is maintained.
Each of these represents one of the 3-D molecular structures shown in the Jmols in Figure 1. You should compare the projection formulas with the ball-and-stick Jmols, trying to visualize the flat projections in three dimensions.
Clearly we could go on to chains of four, five, six, or more carbon atoms by adding more methylene groups to the propane molecule. The first 10 compounds whose structures may be derived in this way are listed in the following table. They are called normal alkanes or straight-chain alkanes, indicating that all contain a single continuous chain of carbon atoms and can be represented by a projection formula whose carbon atoms are in a straight line.
Table \(\PageIndex{1}\) First 10 Alkanes
| Name | Molecular Formula | 3D Model | Condensed Structural Formula | Boiling Point (oC) |
|---|---|---|---|---|
| Methane | CH4 |  |
CH4 | -162 |
| Ethane | C2H6 | 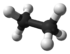 |
CH3CH3 | -89 |
| Propane | C3H8 | 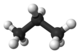 |
CH3CH2CH3 | -42 |
| n-Butane* | C4H10 | 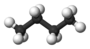 |
CH3(CH2)CH3 | -0.5 |
| n-Pentane* | C5H12 | 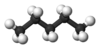 |
CH3(CH2)3CH3 | 36 |
| n-Hexane* | C6H14 | 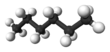 |
CH3(CH2)4CH3 | 69 |
| n-Heptane* | C7H16 | 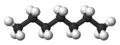 |
CH3(CH2)5CH3 | 98 |
| n-Octane* | C8H18 | 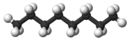 |
CH3(CH2)6CH3 | 126 |
| n-Nonane* | C9H20 | 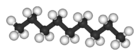 |
CH3(CH2)7CH3 | 151 |
| n-Decane* | C10H22 |  |
CH3(CH2)8CH3 | 174 |
*Please see next section.
Note that all the projection formulas in the table have an initial hydrogen atom followed by a number of CH2 groups. The chain ends with a second single hydrogen atom. The general formula H(CH2)nH, or CnH2n+2, may be written, where n is the number of CH2 groups, or the number of C atoms. Propane, for example, has n = 3. Its formula is C3H8 and it is referred to as a C3 hydrocarbon.
Alkane Isomers
The designation n in front of hydrocarbons butane and above designate a normal straight-chain isomer, with no carbon branches. In addition to the straight chain shown for normal butane, a branched chain, in which some carbon atoms are linked to more than two other carbons, is possible. The projection formula for the branched-chain compound 2-methylpropane (also called isobutane because it an isomer of butane) is
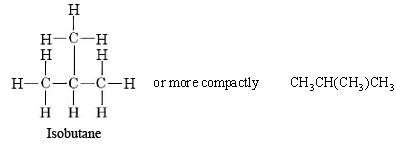
|
Figure \(\PageIndex{4}\) The two isomers of butane, C4H10, n-butane and isobutane, also called 2-methyl-propane. |
Unlike normal butane, which has a straight chain of four carbon atoms, the longest chain in isobutane is only three carbon atoms long. The central of these three atoms is bonded to the fourth carbon. Nevertheless, you can verify from the projection formulas or from the ball-and-stick drawings that both normal butane and isobutane have the same molecular formula, C4H10. The two compounds are isomers, just like ethyl alcohol and dimethyl ether, hence the prefix iso in the name for one of them. Isomers have have the same structural formula, but they often have dissimilar properties. Isobutane, being more compact, has smaller London Force interactions than n-butane, and thus a lower boiling point. The boiling points can be compared in the table of organic compound boiling points. One should note from this table that of all the organic compounds to consider, alkanes, both straight chained and branched, have the lowest boiling points comparatively.
As the number of carbon atoms in an alkane molecule increases, so do the possibilities for isomerism of this kind. There are three isomeric pentanes, all with the formula C5H12, five isomeric hexanes, C6H14, and nine isomeric heptanes, C7H16. The number of possible isomers of tetracontane, C40H82 is larger than 62 million. Thus an inconceivable variety of different molecular structures is possible for compounds containing only carbon and hydrogen atoms connected by single bonds. In crude oil, the most important source of hydrocarbons in the United States, branched-chain and straight-chain alkanes are about equally common.
Another aspect of the behavior of alkane molecules (and other molecules containing single bonds) is not apparent from ball-and-stick illustrations or from projection formulas. Like small children, molecules cannot stop wriggling, and most alkane structures are not rigid. Groups on either side of a carbon-carbon single bond are able to rotate freely with respect to each other. Rotation of one methyl group with respect to the other in ethane, CH3CH3, is shown in Figure 5. While there is free rotation around the carbon bond, certain positions are more stable than others. For the ethane molecule is most stable when the hydrogen atoms on one methyl group are offset from those on the other methyl group(referred to as staggered) and more energy is needed to pass through the eclipsed formation, where the hydrogen atoms of both methyl groups line up. Because of this free rotation, and because they collide with other molecules, alkane molecules are constantly flexing and writhing about their C―C bonds, assuming different shapes (different conformations) all the time. Some feeling for the way in which alkane molecules can adopt a variety of conformations is obtained from the following example.
 Figure \(\PageIndex{5}\) In all alkanes, portions of the molecule can flex or rotate about all the single carbon-carbon bonds. (a) Shown in the animated figure is the rotation of one methyl group, CH3, with respect to the other in a molecule of ethane, CH3CH3. It takes more energy to go through the eclipsed formation, where hydrogen atoms on both methyl groups line up. (b) A movie showing the rotation, vibration and translation of hexane (C6H14). See if you can find where the tail-end CH3 group rotates like the image in part (a). |
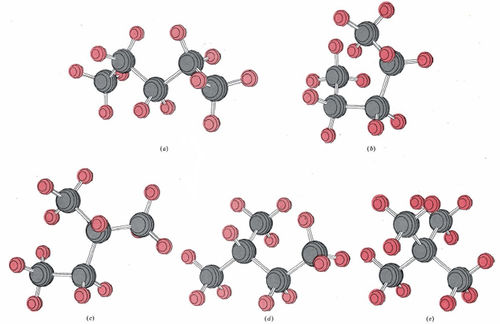
In Figure \(\PageIndex{6}\), five ball-and-stick diagrams labeled (a) through (e) are shown. All five correspond to the formula C5H12 (pentane). Since there are only three isomers of pentane, some of these molecules must correspond to different views or different conformations of the same molecule. Decide which of these diagrams correspond to the same isomer, and which isomer each represents. Draw a projection formula for each isomer.
Solution When given illustrations of molecules in 3D form, it is often helpful to draw them simply in 2D and compare how different atoms bonds to each other. Molecule (a) has five carbon atoms in a single continuous sequence. It corresponds to
Careful inspection of (b) reveals that again the five carbon atoms form a single chain. No carbon atom is joined to more than two others. Molecule (b) is thus also n-pentane, but in a different conformation. Molecule (c) does have a carbon atom joined to three others. The longest chain is four carbon atoms long, and an additional carbon atom is attached to the second carbon atom. The projection formula is accordingly
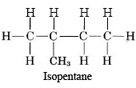
Molecule (d) is also isopentane, while molecule (e) corresponds to the third isomer, called neopentane:
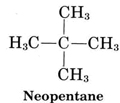
From ChemPRIME: 8.5: Alkanes


