8.10: Unsaturated Hydrocarbons
- Page ID
- 49446
Two important families of hydrocarbons which are not found in petroleum are the alkenes (also called olefins) and the alkynes (also called acetylene). As can already be seen, there are often two names for simple organic compounds. Since 1930 the International Union of Pure and Applied Chemistry(IUPAC) has developed a systematic method for naming all organic compounds, but many of the earlier names still survive, particularly among industrial chemists. Where appropriate, both names will be given, the older name in parentheses. Alkene molecules are similar to alkane molecules, except that they contain a carbon-carbon double bond (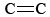 ) and two fewer H atoms. They thus have the general formula CnH2n. Alkyne molecules contain triple bonds (
) and two fewer H atoms. They thus have the general formula CnH2n. Alkyne molecules contain triple bonds ( ) and have four H atoms less than the corresponding alkane. Their general formula is thus CnH2n–2. Compounds containing double or triple bonds are often referred to collectively as unsaturated compounds. Because of their multiple bonds, alkenes and alkynes are usually more chemically reactive than alkanes and aromatic hydrocarbons.
) and have four H atoms less than the corresponding alkane. Their general formula is thus CnH2n–2. Compounds containing double or triple bonds are often referred to collectively as unsaturated compounds. Because of their multiple bonds, alkenes and alkynes are usually more chemically reactive than alkanes and aromatic hydrocarbons.
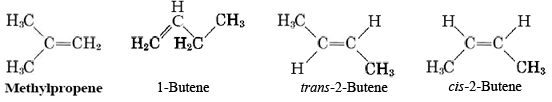
The presence of a double or triple bond in the molecule opens up many more possibilities for isomerism than is the case for alkanes. There are usually several alternative locations for the multiple bond, and in the case of a double bond there is the possibility of cis-trans isomerism. Thus while there are only two alkane molecules possible with four carbon atoms, four alkene molecules are possible:
The bent-bond picture makes it easy to explain several characteristics of double bonds. As noted in Chemical Bonding - Electron Pairs and Octets, the distance between two atomic nuclei connected by a double bond is shorter than if they were connected by a single bond. In the case of carbon-carbon bonds, for example, the 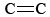 distance is 133 pm, while the C—C distance is 156 pm. This makes sense when we realize that each bent bond extends along a curved path. The distance between the ends of such a path (the C nuclei) is necessarily shorter than the path itself.
distance is 133 pm, while the C—C distance is 156 pm. This makes sense when we realize that each bent bond extends along a curved path. The distance between the ends of such a path (the C nuclei) is necessarily shorter than the path itself.
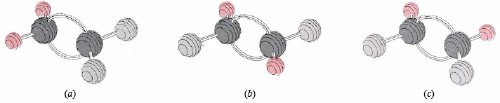
Another characteristic of double bonds is that they make it difficult to twist one end of a molecule relative to the other. This phenomenon usually is called a barrier to rotation. Such a barrier accounts for the fact that it is possible to prepare three different compounds with the formula C2H2F2. Their structures are shown in Figure 2. Structure (a) is unique because both F atoms are attached to the same C atom, but (b) and (c) differ only by a 180° flip of the right-hand 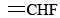 group. If there were no barrier to rotation around the double bond, structures (b) and (c) could interconvert very rapidly whenever they collided with other molecules. It would then be impossible to prepare a sample containing only type (b) molecules or only type (c) molecules.
group. If there were no barrier to rotation around the double bond, structures (b) and (c) could interconvert very rapidly whenever they collided with other molecules. It would then be impossible to prepare a sample containing only type (b) molecules or only type (c) molecules.
Since they have the same molecular formula, (a), (b) and (c) are isomers. Structure (b) in which the two F atoms are on opposite sides of the double bond is called the trans isomer, while structure (c) in which two like atoms are on the same side is called the cis isomer. It is easy to explain why there is a barrier to rotation preventing the interconversion of these cis and trans isomers in terms of our bent-bond model. Rotation of one part of the molecule about the line through the C atoms will cause one of the bent-bond electron clouds to twist around the other. Unless one-half of the double bond breaks, it is impossible to twist the molecule through a very large angle.
Although alkenes are not present in crude petroleum, they are produced in large quantities in petroleum refining. Many of the hydrocarbons in crude oil have very long chains and are solids or thick syrupy liquids at ordinary temperatures because of their relatively large intermolecular forces. The most important petroleum product, gasoline, requires molecules containing from 6 to 12 carbon atoms. These can be obtained by heating the longer-chain compounds. These big molecules writhe around so fast at higher temperatures that they “crack” or break into smaller fragments. Usually a catalyst is added to speed up the reaction, which is called catalytic cracking. When cracking occurs, one alkene and one alkane molecule are produced:
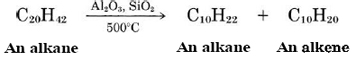 The most important alkenes from an industrial point of view are the two simplest: ethene (ethylene),
The most important alkenes from an industrial point of view are the two simplest: ethene (ethylene), 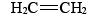 and propene (propylene),
and propene (propylene), 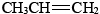 . Almost 1.11 × 1011 kg of ethene and 6.9 × 1010 kg of propene were consumed worldwide in 2006 [1]. Both are used in the manufacture of plastics. They are also raw materials for production of detergents, antifreeze, elastics, and lubricating oils.
. Almost 1.11 × 1011 kg of ethene and 6.9 × 1010 kg of propene were consumed worldwide in 2006 [1]. Both are used in the manufacture of plastics. They are also raw materials for production of detergents, antifreeze, elastics, and lubricating oils.
Among the alkynes only the two simplest are of any industrial importance. Both ethyne (acetylene), and propyne (methyl acetylene),
and propyne (methyl acetylene),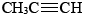 , are used in welding and steel cutting where they are burned together with pure oxygen gas in an oxyacetylene torch. As supplies of petroleum dwindle, though, acetylene may become more important as a starting material for the manufacture of other chemicals, since it can be made from coal.
, are used in welding and steel cutting where they are burned together with pure oxygen gas in an oxyacetylene torch. As supplies of petroleum dwindle, though, acetylene may become more important as a starting material for the manufacture of other chemicals, since it can be made from coal.
- Thomasson, A. "CMAI Announces Completion of Olefins World Analyses; World Light Olefins Analysis and World Butadiene Analysis." Chemical Market Associates, Inc. 5 December 2006. www.cmaiglobal.com/Marketing/...WLOA_WBA07.pdf


