10.6: Photochemical Cycloaddition
- Page ID
- 190071
As noted previously, the Diels Alder reaction is a classic example of a pericyclic reaction.
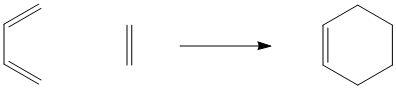
A Diels Alder reaction is sometimes called a [2+4] addition reaction. A 2-carbon unit on one molecule interacts with a 4-carbon unit on another molecule.
In contrast, the addition of one regular alkene to another regular alkene would be called a [2+2] addition reaction. If this reaction occurred, two alkenes would come together to form a four-membered ring.
However, [2+2] addition reactions don't occur without special circumstances. There are a couple of reasons why, and you may be able to suggest some at this point.
You might say that the four-membered ring would be much more strained than the six-membered ring formed by the Diels Alder reaction. That is true, but it may not be reason enough to prevent the reaction from happening. Four-membered rings do occur in nature despite their strain energy.
You might also say that the benzene-like transition state that stabilizes the pathway through a Cope or Diels Alder reaction isn't possible in a [2+2] addition. In fact, the transition state would be more like antiaromatic cyclobutadiene. The transition state would be very high in energy.
Another problem shows up if we look at the orbital interactions in a [2+2] addition reaction. The HOMO on one alkene and LUMO on the other alkene do not overlap so that bonds can form between the two ends. If the p orbitals on one end are in phase, the p orbitals on the other end must be out of phase. The concerted reorganization of bonding possible for the Diels Alder reaction can't happen here.
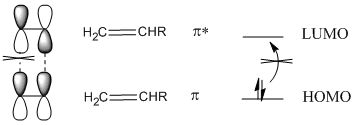
In fact, there is a way around that problem. Irradiating an alkene with UV light leads to promotion of an electron from the HOMO to the LUMO. The alkene is now in an "excited state".
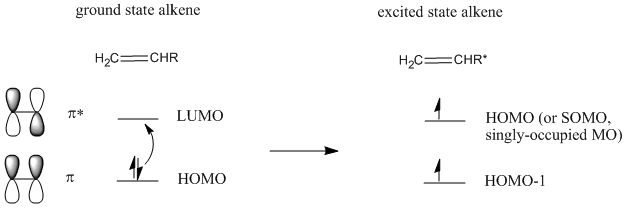
This does not happen with 100% efficiency, so only some of the alkenes will become excited. In the excited state alkene, the LUMO now resembles the HOMO of the ground state alkene. Because of the matching symmetry between these orbitals, the addition reaction can proceed.
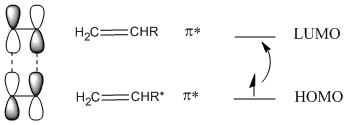
A [4+2] reaction is sometimes referred to as "thermally-allowed", whereas a [2+2] addition is sometimes referred to as "photolytically-allowed." This distinction refers to the need for electronic excitation to accomplish the latter type of reaction.


