9.1: Solutions to Selected Problems
- Page ID
- 203961
Exercise 9.2.1:
- blue and red; green
- green; red
- blue; orange
- violet
Exercise 9.2.2:
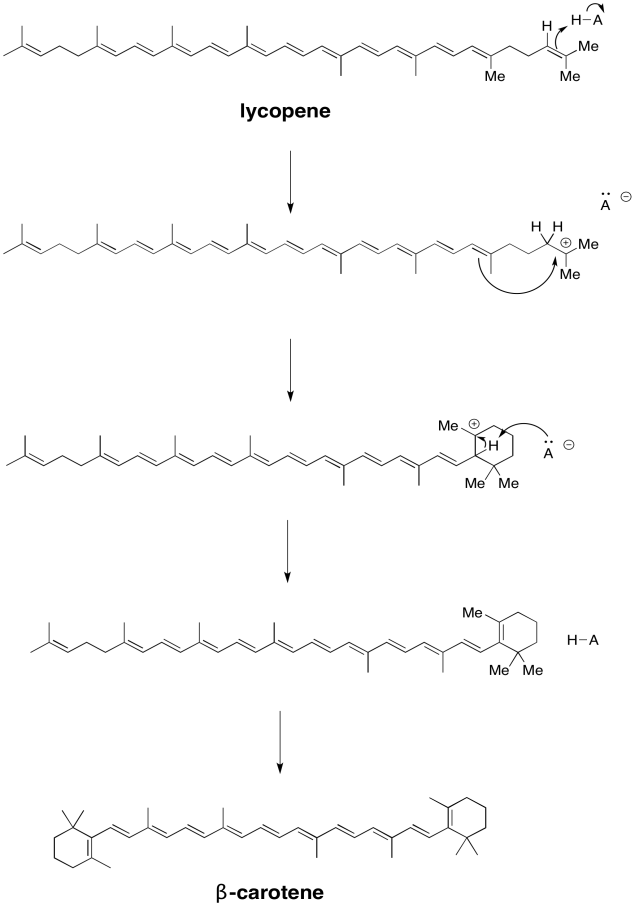
Exercise 9.2.3:
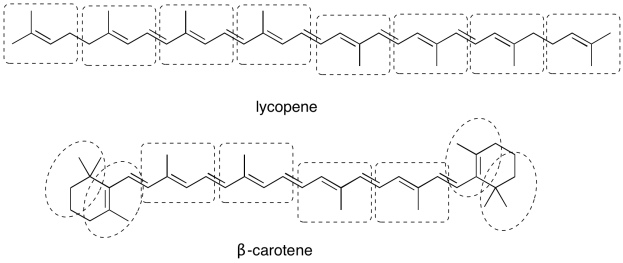
Exercise 9.3.1:
a)
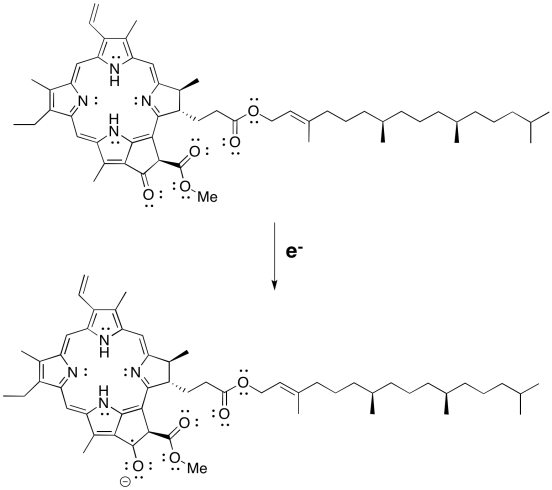
b)
Exercise 9.3.2:
Pheophytin b is probably more electrophilic because of the extra formyl (-HC=O) group. At first glance, it would be expected to have a more positive reduction potential.
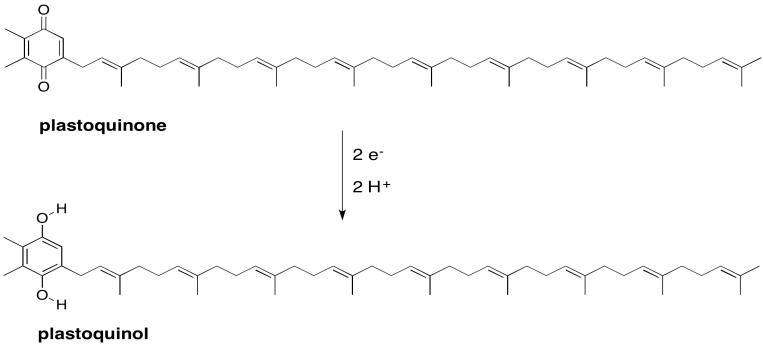
Exercise 9.3.3:
Exercise 9.3.4:
\[\Delta E = E^{o} (red) - E^{o} (ox) = 1.3 - 1.229 V = 0.07 V \nonumber\]
Exercise 9.3.5:
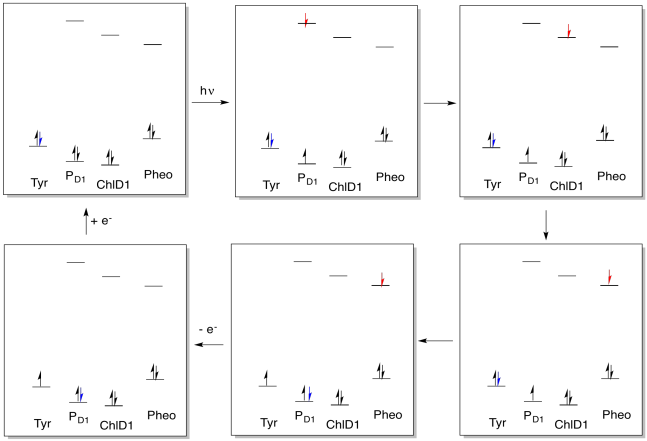
Exercise 9.4.1:
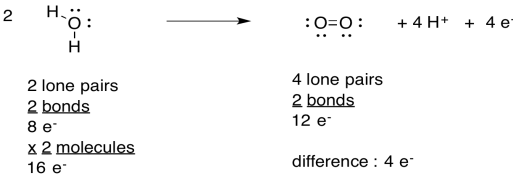
Exercise 9.4.2:
a) Fe(II, III) active b) Mg(II) inactive c) Cu(I, II) active d) Li(I) inactive
e) Zn(II) inactive f) Sc(III) inactive g) Co(II, III) active h) Cr(II, III) active
Exercise 9.5.1:
The first pair of plastoquinols recycle two electrons between them, pumping two additional protons via a recycled quinol. The proton output is 1.5 times what it would be without the Q-loop (from 1 proton/electron + 0.5 proton/electron). Now remember that this recycled quinol also delivers two electrons, one of which is recycled. To get a second electron to generate a new recycled quinol, we would have to go through the entire, two-new-plastoquinol cycle again. We would get another two protons but it would have required eight electrons to get there, or 0.25 protons output per electron input. The proton output would rise to 1.75 times what it would be without the Q-loop (from 1 proton/electron + 0.5 proton/electron + 0.25 proton/electron). A series will result if we keep going. The series is 1 + 1/2 + 1/4 + 1/8 + 1/16 + ..... + 1/2n, and it will converge to 2. Therefore, the Q-loop doubles the number of protons pumped across the membrane.
Exercise 9.5.2:
The major factor appears to be charge stabilisation. Suppose both iron atoms start as Fe(III). They have an overall 6+ charge. The two sulfides are each 2-, for an overall Fe2S2 core charge of 2+. If ligated by four cysteines (Cys-S-), then the overall charge of the coordination compound is 2-. Addition of an electron will result in an increase in negative charge, to 3-. Now, that was starting from the fully oxidised state. If instead we start in a mixed Fe(II/III) oxidation state, then addition of an electron results in a 4- charge on the coordination complex. That charge buildup will be energetically difficult.
If ligated by two cysteines (Cys-S-) and two neutral histidines, then the overall charge of the coordination compound is 0. Addition of an electron will result in a negative charge, 1-. If instead we start in a mixed Fe(II/III) oxidation state, then addition of an electron results in a 2- charge on the coordination complex. That lower charge buildup required in this case will be energetically less difficult.
Exercise 9.5.3:
Copper is further to the right in the periodic table and is consequently more electronegative than iron. That is the major reason that copper is below iron in the activity series or metals. On that basis alone (and ignoring any differences in the coordination environment), we might expect a more positive reduction potential on a copper ion than an iron ion. Copper would therefore be a better electron acceptor, and would be useful in creating a greater driving force for electron transfer later in the electron transport chain.
Exercise 9.5.4:
- With only one anionic donor, the charge of the complex that contains Cu(II) would be 1+.
- With one anionic donor, the charge of the complex that contains Cu(I) would be 0.
- Reduction of plastocyanin results in a decrease of charge, whereas reduction of the FeS cluster results in an increase of charge. That factor alone could make plastocyanin easier to reduce, with a more positive reduction potential.
Exercise 9.6.1:
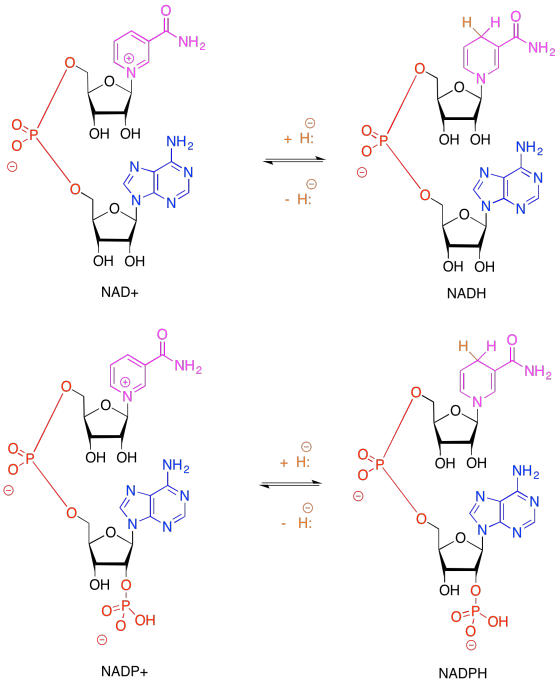
Exercise 9.6.2:
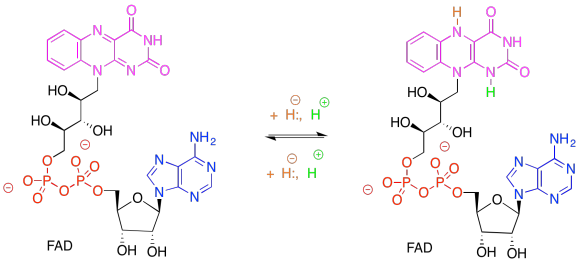
Exercise 9.6.3:
Remember, NADP+, like NAD+, is solely a 2-electron oxidising agent. It can only receive a pair of electrons associated with a hydride. Ferredoxin, via an FeS cluster, can only give one electron at a time. An adapter is needed between these two carriers. FAD can accept either 1 or 2 electrons, proceeding to either FADH. radical (stabilised via extensive resonance delocalisation) or to FADH2.
Exercise 9.6.4:
- Releasing antennae molecules from the light harvesting complex in photosystem II would decrease both the number of photons captured and the number of electrons sent into the electron transport chain. Conversely, in a "chromophore pool" model, releasing these molecules from photosystem II would make them more available for uptake by photosystem I, resulting in greater absorption of photons there, and greater capacity to accept incoming electrons. If regulated, these two systems should be able to level out at an identical rate of photon absorption.
- Kinases phosphorylate the hydroxy side chains in serine residues (and sometimes threonines and tyrosines). That covalent modification results in a change in the charge of the side chain from neutral to anionic. Significant conformational changes in the protein can be expected to result. It is likely that this conformational change results in looser binding of the antennae molecules in the light harvesting complex of photosystem II.
Furthermore, kinase appears to promote tighter binding of antennae molecules at photosystem I, presumable through a conformational change there. The result is a shift of these molecules from the available pool, such that additional chromophores become available in photosystem I and fewer are retained in photosystem II, until photon absorption at the two sites is equal.
c) In fact, phosphatase, which is the complementary enzyme to kinase, is activated when photosystem I has high activity compared to photosystem I. Phosphatases remove phosphate groups from serines and related residues. The result would be tighter binding of antennae molecules at photosystem I and looser binding of antennae molecules at photosystem II, so that light absorption could be evened out between the two sites.
The use of phosphatases and kinases to effect opposite objectives in regulation is common in a number of biochemical systems.
Exercise 9.8.1:
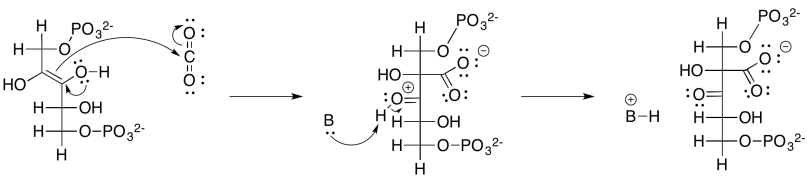
Exercise 9.8.2:
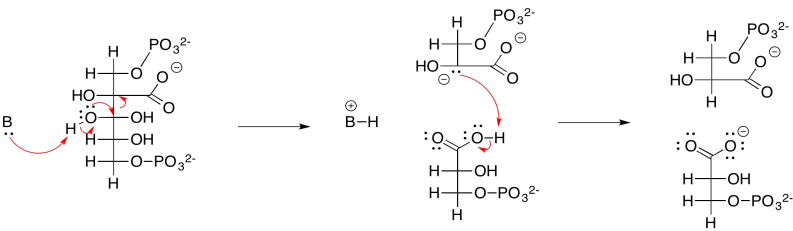
Exercise 9.8.3:
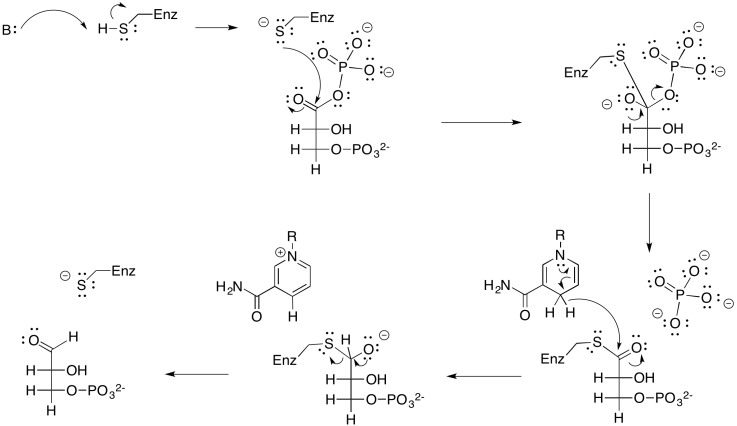
Exercise 9.8.4:
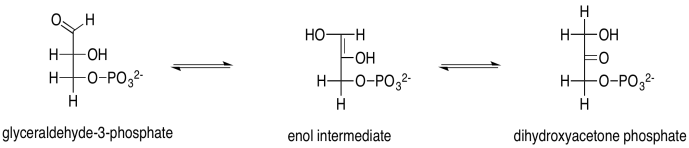
Exercise 9.8.5:
a)
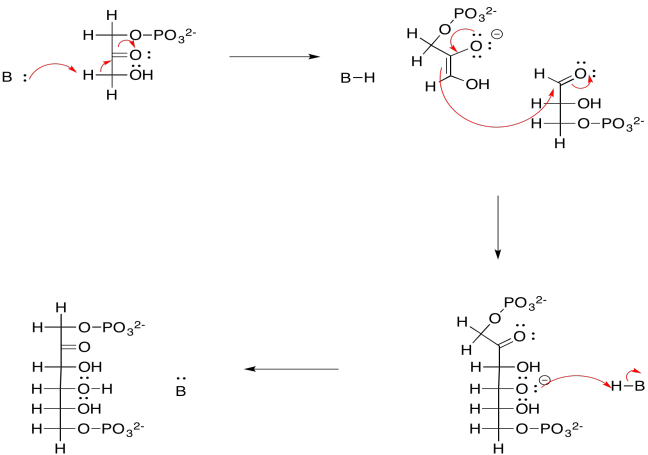
b)
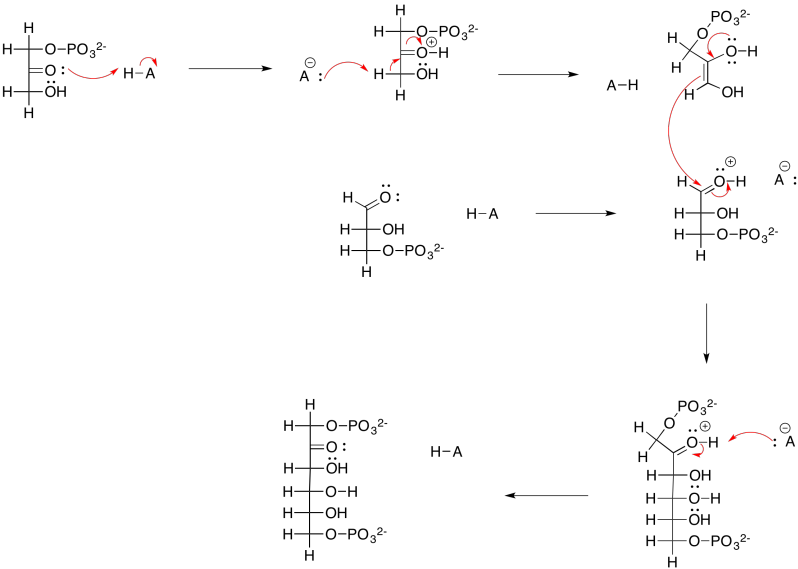
Exercise 9.8.6:
a)
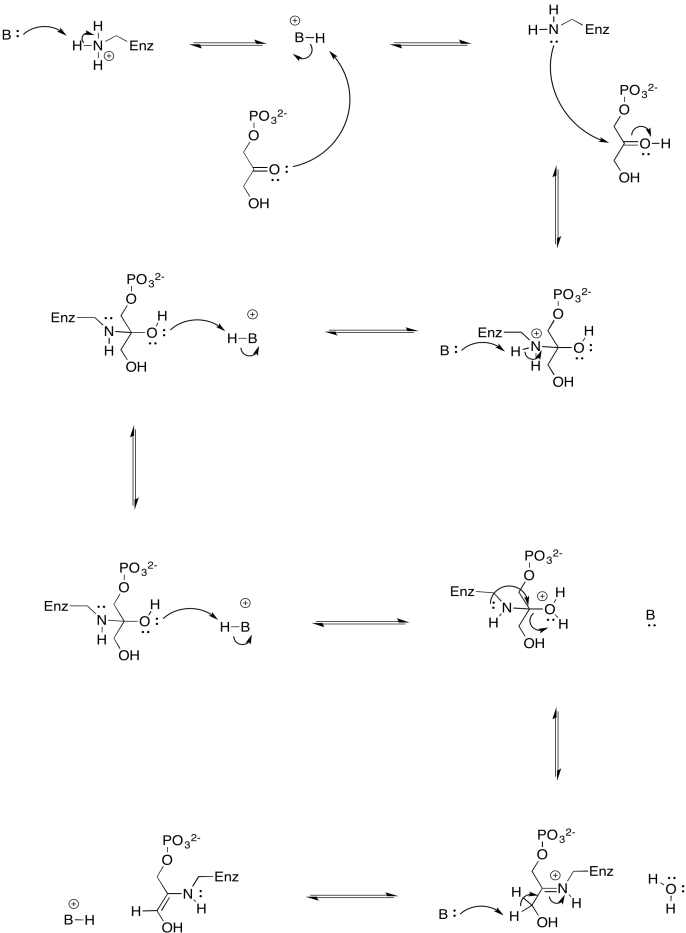
b)
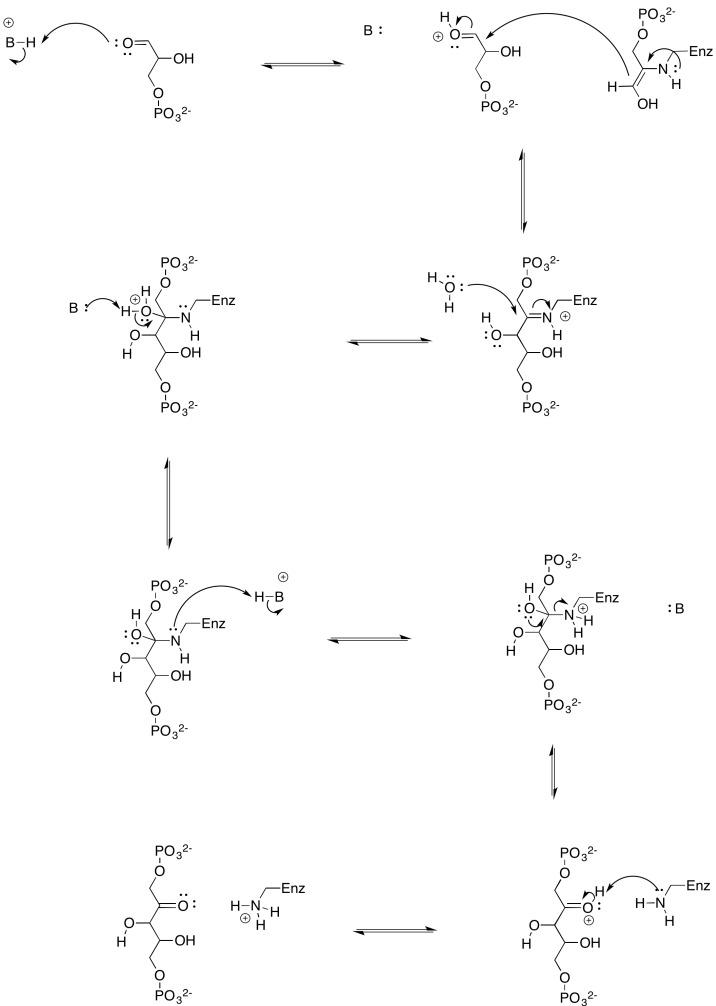
Exercise 9.8.7:
The regular enol pathway is driven by pi-donation from an oxygen atom. The enamine pathway is driven by pi-donation from a nitrogen. Nitrogen is less electronegative and a better pi-donor than oxygen, so the enamine pathway is faster.
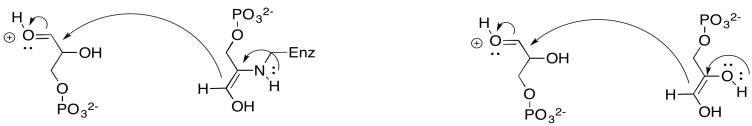
Exercise 9.8.8:
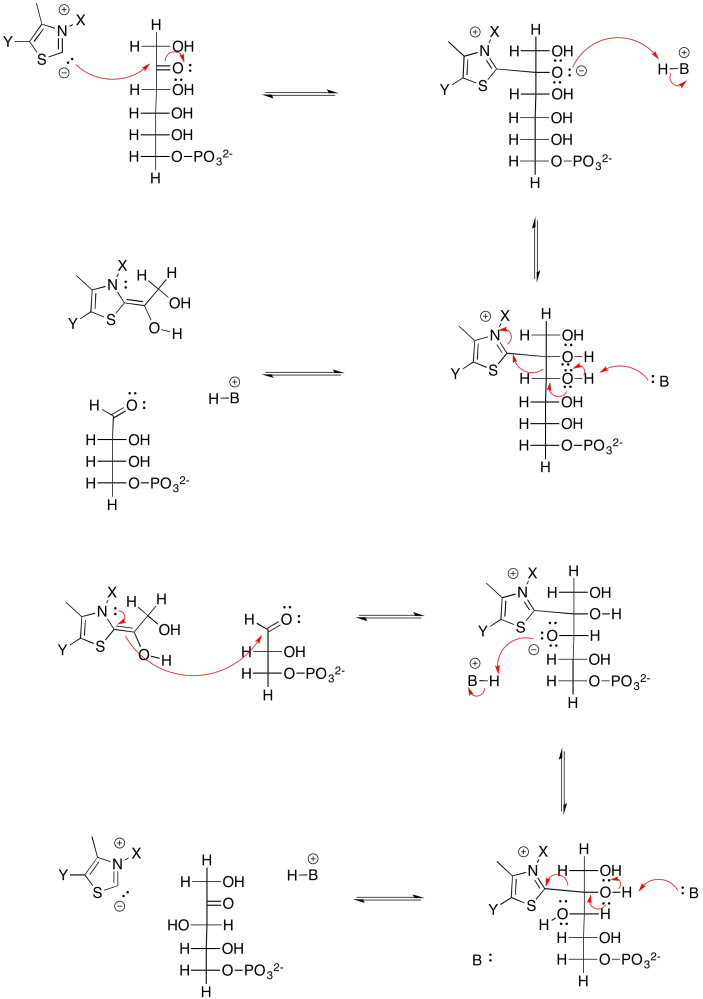
Exercise 9.8.9:
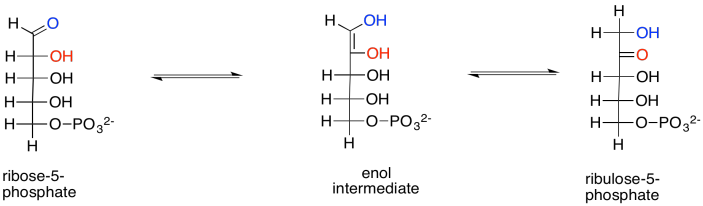
Exercise 9.8.10:
Reaction that are in equilibrium are often under substrate control. Because the enzyme can catalyse the reaction in both directions, the direction of the reaction driven by the enzyme is controlled by the relative amount of reactants on either side of the reaction. For example, if the xylulose concentration greatly increased, then the amount of xylulose bound by the enzyme would also increase, so the enzyme would shift xylulose into ribulose and thereby maintain equilibrium. If the ribulose concentration greatly increased, then more ribulose would be bound by the enzyme, and the ribulose would be converted into xylulose.
Exercise 9.9.1:
a)
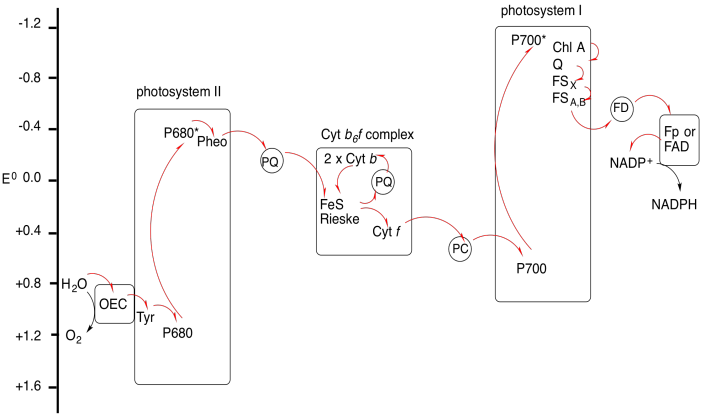
Exercise 9.9.2:



