8.5: Atmospheric Photochemistry- Ozone
- Page ID
- 190056
There are lots of ways that light plays an important role in the world. Life on earth is heavily dependent on photosynthesis. Energy from the sun can be harvested and used to make ATP, which can then be used for carbohydrate synthesis. The energy found in light, however, can also be very damaging. In fact, ultraviolet light would make life impossible on earth were it not for the intercession of the high-atmosphere ozone layer. By undergoing reactions that absorb UV light, the molecules involved in the ozone cycle block much of the most harmful sunlight from reaching the earth's surface.
The ozone cycle is a series of reactions involving allotropes of oxygen. Oxygen can be present as oxygen atom, O; as oxygen molecule, O2; or as ozone, O3. Of course, dioxygen, or molecular oxygen, is by far the most common of these forms, constituting about 20% of our atmosphere.
That's where we will start. There is plenty of dioxygen in our atmosphere, and so photons from the sun encounter these molecules pretty frequently. If the photons have sufficient energy, they can break the O-O bond, forming oxygen atoms.
\[\ce{O2 + energy -> 2O} \nonumber\]
That photolysis reaction (meaning, "broken by light") leads to a buildup of oxygen atoms in the upper atmosphere. Of course, these oxygen atoms are likely to encounter oxygen molecules, which are very abundant. When they do, they can undergo a bond-forming reaction, to make ozone. This reaction is purely bond-forming, and it is exothermic.
\[\ce{O + O2 -> O3 + energy} \nonumber\]
There are also a couple of reactions that cause removal of ozone from the atmosphere. The first is the reaction of ozone with ultraviolet light. The energy from the sunlight can break the O-O bond in ozone, converting it back into dioxygen and oxygen atom.
\[\ce{O3 + energy -> O + O2} \nonumber\]
In addition, as ozone and oxygen atom build up, collisions between these two species becomes increasingly likely. If that happens, an oxygen atom abstraction is an obvious outcome, in which two dioxygen molecules are the products. This reaction involves both bond-breaking and bond-making, but the reaction is overall exothermic.
\[\ce{O3 + O -> 2O2 + energy} \nonumber\]
Notice those two energy-consuming reactions. One of them occurs in the ozone creation phase and one in the ozone removal phase. Together, these reactions contribute to the consumption of ultraviolet light as it passes through the stratosphere, so that less ultraviolet light reaches the earth's surface below. However, the two reactions are on opposing sides of a cycle. Consumption of some sunlight leads to ozone formation, but consumption of sunlight also leads to ozone destruction.
The thermodynamics of the ozone cycle are illustrated below. Again, two of the reactions are endothermic, whereas two others are endothermic.
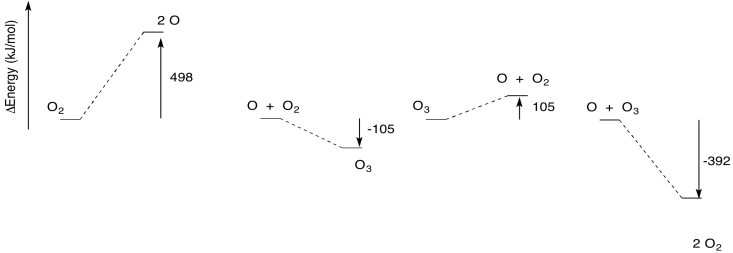
The endothermic steps actually require more energy than is implied in the picture above. As in any reaction, it usually isn't sufficient to supply enough energy to get from one side of the reaction to the other. There is also an energy barrier to overcome. That's part of the reason these reactions need ultraviolet light.
The first reaction requires light of wavelengths shorter than about 240 nm.
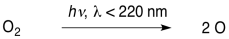
The second reaction requires light of wavelengths shorter than about 325 nm.
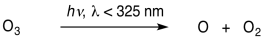
Exercise \(\PageIndex{1}\)
Why is there an upper limit to the wavelength of light capable of inducing these reactions?
- Answer
-
The longer the wavelength, the lower the energy. Photons of wavelength longer than 240 nm would not have enough energy to overcome the barrier for the dioxygen-cleaving reaction.
Exercise \(\PageIndex{2}\)
Calculate the energy, in J, of the following photons:
- 220 nm
- 325 nm
- Answer a
-
According to the Planck-Einstein relation:
E = hν
or, since ν = c / λ
E = hc / λ
in which h = Planck's constant = 6.625 x 10-34 Js,
c = speed of light = 3.0 x 108 m/s,
ν = frequency,
λ = wavelength.
a) E = hc / λ
E = (6.525 x 10-34 Js)(3.0 x 108 m/s) / (220 nm)(10-9 m/nm)
E = 9.03 x 10-19 J
- Answer b
-
b) E = hc / λ
E = (6.525 x 10-34 Js)(3.0 x 108 m/s) / (325 nm)(10-9 m/nm)
E = 6.12 x 10-19 J
Exercise \(\PageIndex{3}\)
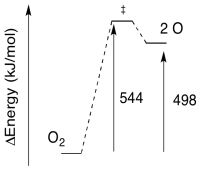
- Given the wavelength of light needed for the photolysis of O2, calculate the barrier to the reaction, in kJ/mol.
- Sketch a reaction progress diagram for the reaction.
- Answer a
-
Remember, the mole is the conversion unit from the molecular scale to the macroscopic scale.
a) E = (8.28 x 10-19 J / photon)(6.02 x 1023 photons/mol)
E = 543,770 J/mol
E = 544 kJ/mol
- Answer b
-
b)
Exercise \(\PageIndex{4}\)
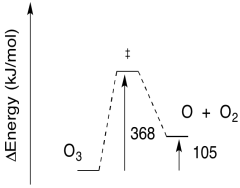
- Given the wavelength of light needed for the photolysis of O3, calculate the barrier to the reaction, in kJ/mol.
- Sketch a reaction progress diagram for the reaction.
- Answer a
-
a) E = (6.12 x 10-19 J / photon)(6.02 x 1023 photons/mol)
E = 368,146 J/mol
E = 368 kJ/mol
- Answer b
-
b)
Exercise \(\PageIndex{5}\)
Given the principle of microscopic reversibility, calculate the barrier to the following reactions, in kJ/mol.
- \(\ce{2O -> O2}\)
- \(\ce{O + O2 -> O3}\)
- Answer a
-
The reactions must take the same pathway, and go over the same barrier, forward and back.
a) The reverse barrier: E = 544 - 498 kJ/mol
E = 46 kJ/mol
- Answer b
-
b) The reverse barrier: E = 368 - 105 kJ/mol
E = 263 kJ/mol
Ozone is clearly at a higher energy than dioxygen. The fact that appreciable amounts exist in the upper atmosphere can be attributed to the constant input of energy from the sun. This is an example of an environmental steady state. The ratio of ozone to oxygen, although still pretty low even in the stratosphere, is elevated above its natural equilibrium value because energy is constantly being added, pushing the system uphill in energy.
The Problem with Chlorine
In 1974, Sherwood Rowland, a professor of chemistry at the University of California, Irvine, and Mario Molina, a Mexican post-doctoral associate in his laboratory, authored a paper in the journal Nature outlining how chlorofluorocarbons (CFCs) could play a role in interfering with the ozone cycle. CFCs at that time were an economically important compound because they were a non-toxic, highly effective refrigerant used in millions of homes and businesses worldwide. Rowland and Molina shared the 1995 Nobel Prize in Chemistry with Paul Crutzen, a Dutch citizen working at the Max Planck Institute in Germany, who similarly reported the effects of nitrogen oxides on the ozone cycle.
The problem with CFCs is really the fact that, in the upper atmosphere, they can break down to form chlorine atoms. For example, freon-12, or CF2Cl2, can absorb ultraviolet light, resulting in cleavage of the C-Cl bond.
Chlorine atom in the stratosphere interferes with the ozone cycle by consuming both ozone and, indirectly, a key intermediate in ozone formation, oxygen atom.
\[\ce{O3 + Cl -> ClO + O2} \nonumber\]
\[\ce{ClO + O -> O2 + Cl} \nonumber\]
What makes the role of chlorine particularly problematic is that, in addition to wiping out ozone and an intermediate needed to replace it, the chlorine is regenerated at the end, so it is able to go back into the system and do it all over again. It is destructive to ozone, and with catalytic efficiency.
After environmental regulations were introduced mandating an eventual industry-wide phase-out of CFCs, industry was able to respond with the development of chlorine-free substitutes that were still non-toxic and non-flammable (both important for household use). Hydrofluorocarbons are the major type of replacement that has been developed for use today. There are other compounds commonly used as refrigerants, but they have significant drawbacks. Ammonia is toxic, and hydrocarbons are very flammable.
There is an additional consideration that researchers must take into account in looking for CFC replacements, however: lifetime. If a molecule lasts too long in the atmosphere, there is the risk of a long-term buildup that may result in additional environmental consequences. The fact that chlorocarbons and fluorocarbons contain polar C-Cl and C-F bonds means that they will absorb infrared radiation strongly, so they will act as greenhouse gases. That's part of the reason hydrofluorocarbons are used. The C-H bond in a hydrofluorocarbon is easily broken by atmospheric hydroxy radical, because of the strength of the new O-H bond that is formed. That event provides for a decomposition pathway that removes the molecule from the atmosphere more quickly.


