6.10: Living Radical Polymerisation
- Page ID
- 202842
Chain polymerisation reactions result in the efficient conversion of monomers into high molecular weight polymers. However, chain termination events result in a broadening of the polydispersity index of the material. In other words, instead of producing a material composed of molecules that are all about the same molecular weight, a wide range of sizes of molecules result. Some of the chains are very short and others are very long. That's a problem, because the chain length (and the associated property, molecular weight) strongly influence the properties of the material. If the size of the molecules is not controlled, neither are the properties. If the properties are not controlled, the material won't perform reliably in its intended application.
The degree of polymerisation of a polymer is simply the average number of monomers incorporated in each polymer chain. Given the following "feed ratios" (ratios of monomer to initiator), what is the expected degree of polymerisation in each case?
- methyl methacrylate : AIBN 500 : 1
- styrene : benzoyl peroxide 1000 : 1
- acrylonitrile : tert-butyl peroxide 600 : 3
- Answer a
-
Because each initiator breaks in half, forming two radicals, one initiator starts two growing chains. Every initiator will, on average, consume half the monomers, assuming no unexpected chain termination events.
a) DP = 500 monomers / 2 growing chains = 250
- Answer b
-
Because each initiator breaks in half, forming two radicals, one initiator starts two growing chains. Every initiator will, on average, consume half the monomers, assuming no unexpected chain termination events.
b) DP = 1000 monomers / 2 growing chains = 500
- Answer c
-
Because each initiator breaks in half, forming two radicals, one initiator starts two growing chains. Every initiator will, on average, consume half the monomers, assuming no unexpected chain termination events.
c) DP = 600 monomers / 3 growing chains = 200
The measured degree of polymerisation indicates the number average molecular weight of the polymer (Mn). Calculate Mn in each of the following cases.
- polystyrene, with DP = 1250
- polymethacrylamide, with DP = 725
- poly(methyl acrylate), with DP = 1420
- Answer a
-
M0 = molecular weight of monomer in each case. 1 D (Dalton) = 1 amu
a) Mn = DP x M0 = 1250 x 104 D = 130,000 D = 130 kD
Note that end groups were neglected in these calculations; they may contribute a significant percentage of total molecular weight at lower DP. A more accurate calculation would account for the identity of both end groups.
- Answer b
-
M0 = molecular weight of monomer in each case. 1 D (Dalton) = 1 amu
b) Mn = DP x M0 = 725 x 85 D = 61,625 D = approximately 62 kD
Note that end groups were neglected in these calculations; they may contribute a significant percentage of total molecular weight at lower DP. A more accurate calculation would account for the identity of both end groups.
- Answer c
-
M0 = molecular weight of monomer in each case. 1 D (Dalton) = 1 amu
c) Mn = DP x M0 = 1420 x 86 D = 122,120 D = approximately 122 kD
Note that end groups were neglected in these calculations; they may contribute a significant percentage of total molecular weight at lower DP. A more accurate calculation would account for the identity of both end groups.
Alternatively, if the number average molecular weight of the polymer is measured, that result can be used to establish the degree of polymerisation. What is DP in each of the following cases?
- polyacrylonitrile, with Mn = 11,450 D
- poly(vinyl acetate), with Mn = 24,760 D
- polystyrene, with Mn = 927,000 D
- Answer a
-
a) DP = Mn / M0 = 11,450 D / 53 D = 216
- Answer b
-
b) DP = Mn / M0 = 24,760 D / 86 D = 288
- Answer c
-
c) DP = Mn / M0 = 927,000 D / 104 D = 8,914
Living polymerisation refers to processes in which unexpected chain termination does not occur. The chain keeps growing and growing as long as more monomer is supplied. In extremely hardy cases, the term "immortal" polymerisation is sometimes used.
Typically, strategies for living polymerisation involve controlling the reactivity of the intermediates. Frequently, the concentration of the growing chains is kept low. If the concentration of growing chains is kept low, then unexpected side reactions involving the reactive growing chain will be kept to a minimum.
In radical polymerisation, growing chains with radicals at their growing ends will be surrounded by monomers. The radicals devour and enchain the monomers as they move through the reaction medium.
Typical chain-terminating events in radical polymerisation involve the collision of two growing chains. That event could result in head-to-head radical recombination, formation of a double bond via hydrogen abstraction at the head of a chain ("head biting") or formation of a new radical along the backbone of the polymer ("backbiting").
Suppose you have three growing polymer chains. The monomers have molecular weight = 100 D. Each chain is currently 8 repeat units long.
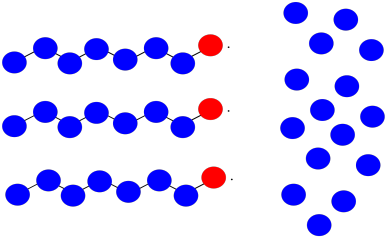
- What is the current molecular weight of each chain?
- If 15 monomers remain in solution, what is the expected degree of polymerisation of each chain, assuming they all grow at the same rate?
- What is the expected average molecular weight?
- What is the expected PDI?
- Suppose two of the chains join together in a termination step. What will be the molecular weight of the new chain?
- If the third chain keeps growing, what molecular weight will it reach?
- What will be the average molecular weight?
- What will be the PDI (assume it's just the ratio of largest to smallest molecular weight)?
- Answer a
-
a) MW = 8 x 100 D = 800 D
- Answer b
-
b) 15 monomers / 3 chains = 5 new monomers / chain
DP = 8 + 5 = 13
- Answer c
-
c) MW = 13 x 100 D = 1,300 D
- Answer d
-
d) All chains are the same length; PDI = 1.0
- Answer e
-
e) MW = 16 x 100 D = 1,600 D
- Answer f
-
f) MW = (8 + 15) x 100 D = 2,300 D
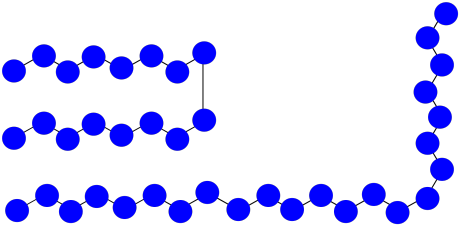
- Answer g
-
g) Mn = (2,300 + 1,600 D) / 2 = 1,950 D
- Answer h
-
h) PDI = 2,300 D / 1,600 D = 1.44
Suppose once again you have three growing polymer chains. The monomers have molecular weight = 100 D. Each chain is currently 8 repeat units long.
This time, one chain abstracts a hydrogen atom from the backbone of another. The first chain is terminated; the second now has two sites of growth. All three sites continue to grow at the same rate.
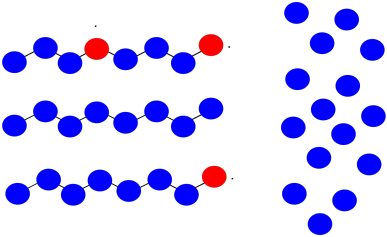
- What will be the molecular weight of each chain?
- What will be the average molecular weight?
- What will be the PDI (assume it's just the ratio of largest to smallest molecular weight)?
- Answer a
-
a) The chain that abstracted the hydrogen: MW = 8 x 100 D = 800 D
The branched chain: MW = 18 x 100 D = 1,800 D
The normal chain: MW = 13 x 100 D = 1,300 D
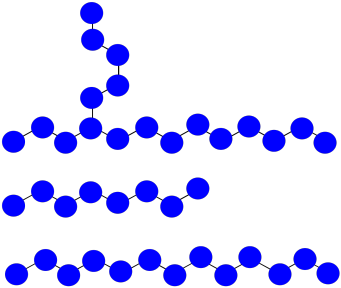
- Answer b
-
b) Mn = (1,800 + 1,300 + 800 D) / 3 = 1,300 D
- Answer c
-
c) PDI = 1,800 / 800 D = 2.25
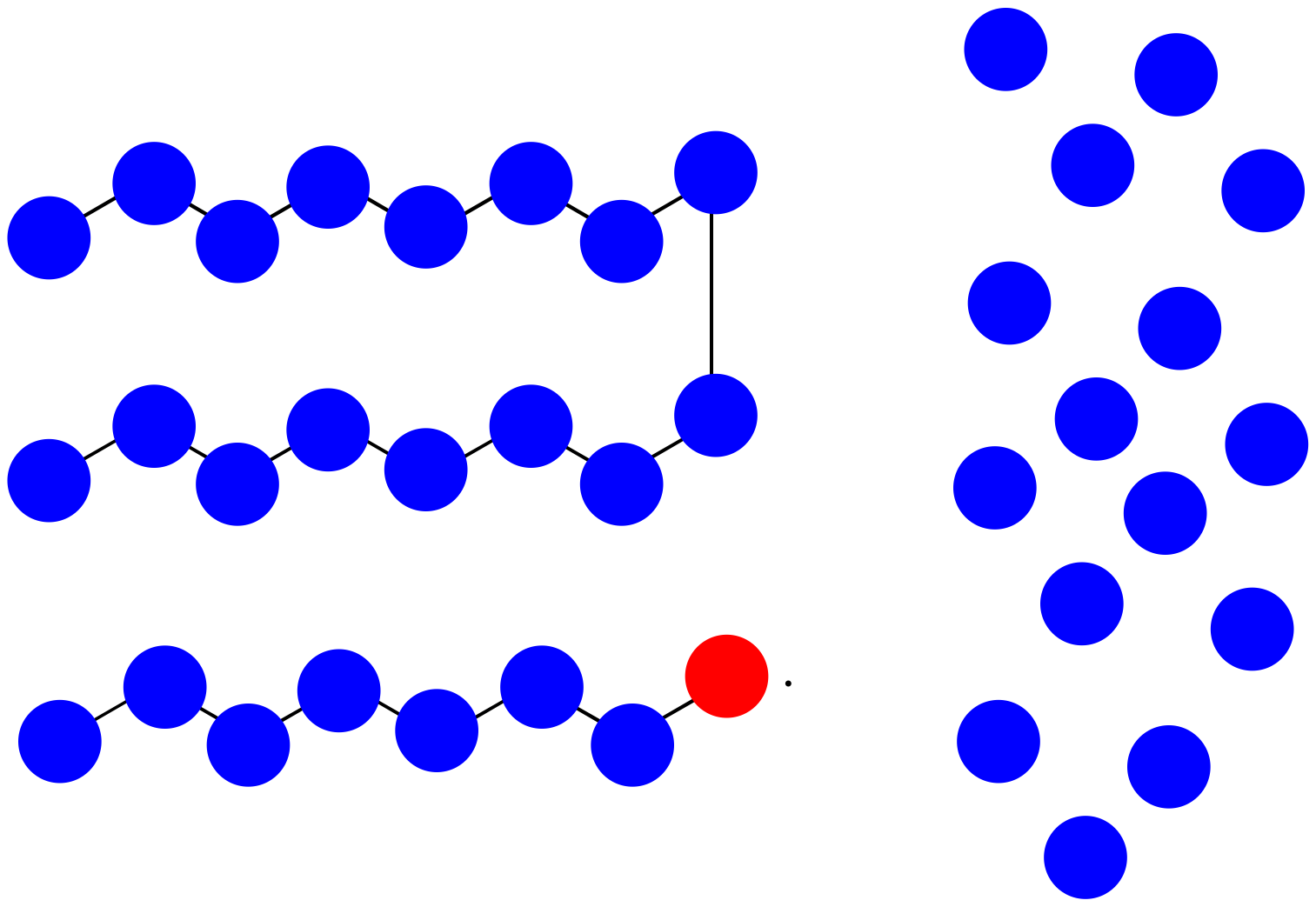
Practically, polymers are purified by precipitation and washing after they are prepared. That means very short oligomers are washed away.
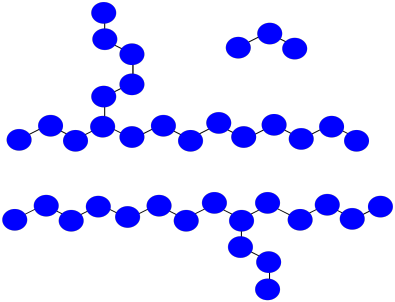
- What is the average molecular weight of the impure mixture shown above, assuming each monomer has molecular weight = 100 D?
- After precipitation and washing, if small oligomers are washed away, what is the avergae molecular weight of the isolated polymer?
- Answer a
-
a) Mn = (1,700 + 1,800 + 300 D) / 3 = 3,800 D / 3 = 1,267 D
- Answer b
-
b) Mn = (1,700 + 1,800 D) / 2 = 3,500 D / 2 = 1,750 D
If the concentration of growing chains is limited, then the probability that any of these events will occur is also limited.
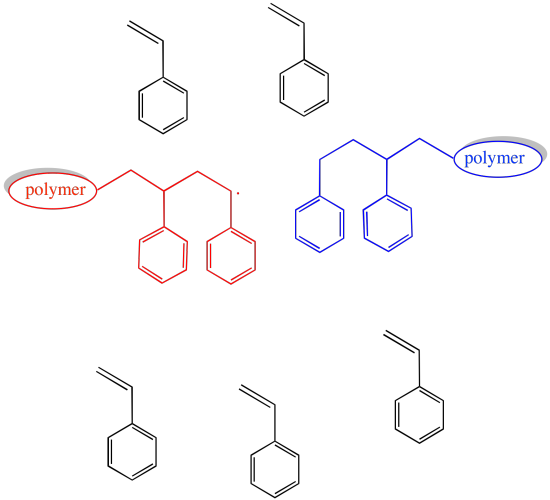
Certainly, the rate of chain growth also slows when the concentration of growing chains is lowered. That is the price to pay for a smooth operation.
The key to living radical polymerisation is to reversibly stop chain growth, sending a polymer chain from an active state into a dormant state. While in the dormant state, the polymer chain is less likely to undergo random chain-termination events. It can't grow, either, until it is brought back into an active state.
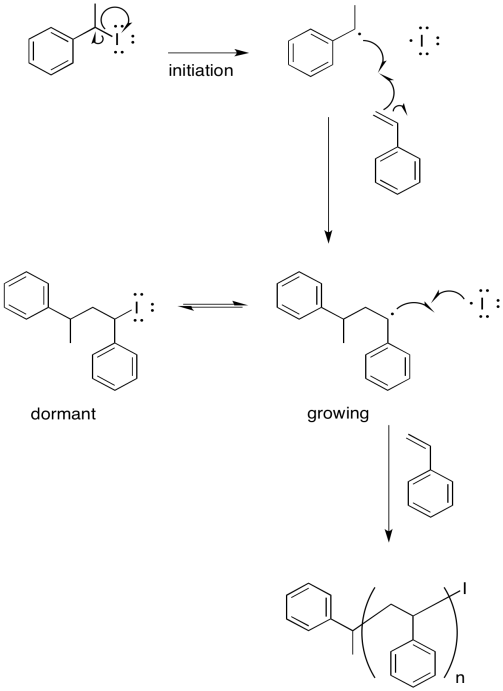
An early attempt at living polymerisation employed an alkyl iodide initiator. Thermal initiation (initiation by heating) resulted in a reactive alkyl radical and an iodine atom.
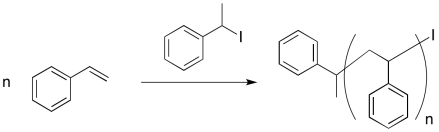
Provide a mechanism for styrene polymerisation, making sure to show the following points:
- initiation
- propagation
- recombination with iodine atom
- a growing chain
- a "dormant" chain that could re-initiate and start growing again, but which is currently safe from random termination
- Answer
-
Researchers at IBM found that polystyrene polymerisation proceeded much more smoothly when TEMPO, a relatively stable radical, was added to the reaction. The reaction also proceeded more slowly, resulting in overall lower molecular weight of the polymer. However, the distribution of molecular weight was much more uniform. All of the chains were of similar sizes, relatively speaking. As a result, the properties of the material were much more controlled.
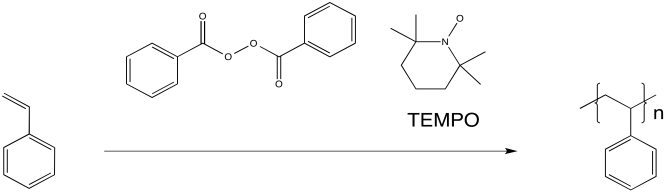
TEMPO helps to control the polymerisation by forming a reversible bond with the growing end of the polymer chain. The radical on TEMPO combines with the radical on the head of the polymer to form a C-O bond. The bond can break again (unusually, for a C-O bond), allowing the polymer chain to resume growing periodically.
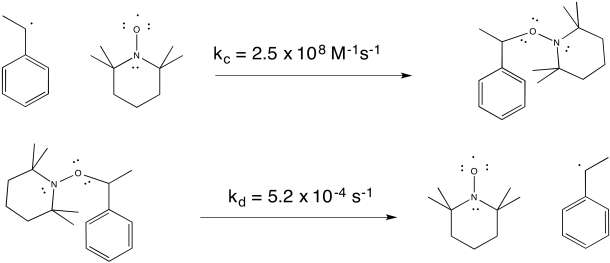
Two of the most common methods of inducing living radical polymerisation are RAFT and ATRP. RAFT stands for radical atom fragmentation polymerisation. Like the TEMPO reaction, it involves a reversible radical recombination to form a covalent bond. RAFT was developed by a group of Australian chemists in the late 1990's, including Enzio Rizzardo, Graeme Moad and San Thang of Australia's national science agency, CSIRO. ATRP stands for atom-transfer radical polymerisation. It was developed in the mid 1990's by Krysztof Matyjaszewski at Carnegie Mellon in Pittsburgh and his post-doctoral associate, Jin-Shan Wang, now at Shanghai Jiao Tong University. An independent discovery of the method was made by Mitsuo Sawamoto at Kyoto University in Japan.
RAFT most commonly employs a thioester (or similar compound) as a chain transfer agent. The chain transfer agent intercepts a growing polymer chain, but does so reversibly.
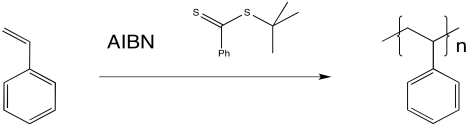
The thioester can react with a radical, placing the growing chain in a dormant state. It can also release a new radical, which can then initiate polymerisation. Eventually, it will regulate the growth of two polymer chains.
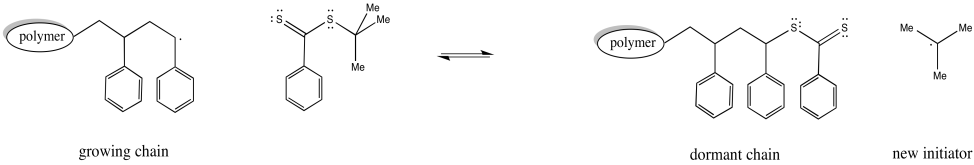
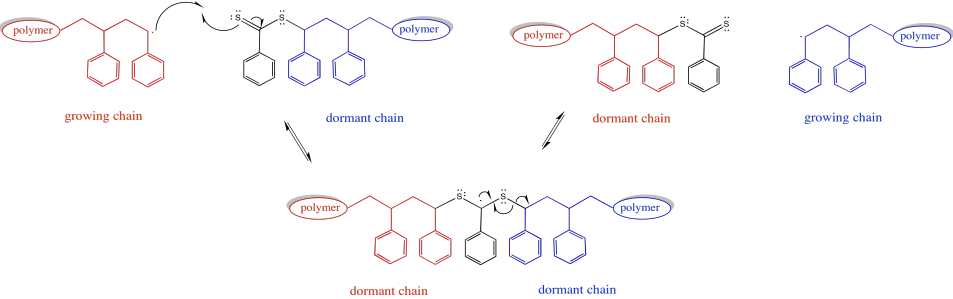
In addition, a third species present in equilibrium holds both chains dormant. The chain transfer agent can reversibly release one of these dormant chains at a time.
Provide a mechanism for conversion of a growing chain into a dormant chain via RAFT.
- Answer
-
In ATRP, a similar process works to keep a fraction of the chains in a dormant state. A key component of this method is a copper(I) complex.
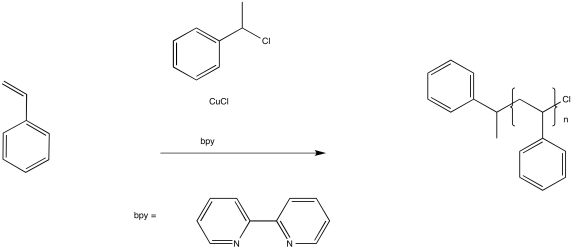
The role of the copper(I) complex is to transfer an electron to an inactive species, producing a copper(II) species and a radical. For example, in initiation of the reaction above, the Cu(I) transfers an electron to the alkyl halide to become Cu(II). In turn, the chlorine atom on the alkyl halide becomes chloride anion, Cl-, and the alkyl portion is left as an initiating radical.

One of the reasons ATRP is so important is that it provides a very reliable and inexpensive way to control polymerisation. In addition, it can be adapted to a wide range of useful processes. For example, Matyjaszeski has developed methods of electrochemically controlling the reaction; polymerisation can literally be turned on and off with a switch. Yusuf Yagci and coworkers at Istanbul Technical University developed a photoinduced ATRP process, in which polymerisation begins when the lights come on and stops when it gets dark. Other researchers, including the Hawker lab at UCSB, have also promoted the utility of this approach.
Provide a mechanism for initiation using ATRP.
- Answer
-
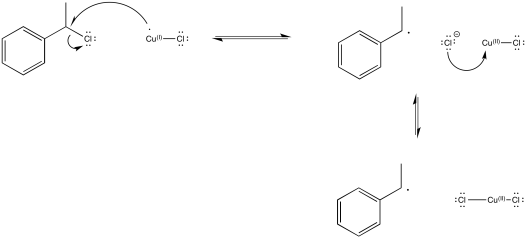
Provide a mechanism for conversion of a growing chain to a dormant chain using ATRP.


