6.7: Radical Substitution
- Page ID
- 202838
There are a couple of classic reactions based on radical chemistry that are often used to illustrate different consequences of the mechanism. One of these reactions is the radical substitution of a halogen atom for a hydrogen atom.
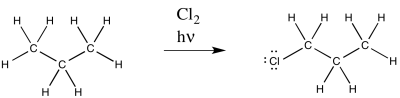
This reaction is commonly observed with chlorine and bromine. Multiple products are possible; only one of these products is shown above.
As a radical reaction, the first step would have to be an initiation step. If the reaction proceeds as written, via the addition of molecular chlorine and light, then initiation would involve direct homolysis of the chlorine-chlorine bond.
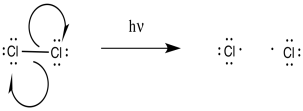
Once the chlorine radicals are present, propagation steps would occur. Given the ultimate replacement of a hydrogen by a chlorine atom, radical abstraction of a hydrogen atom seems likely. That event would lead to the formation of an alkyl radical.
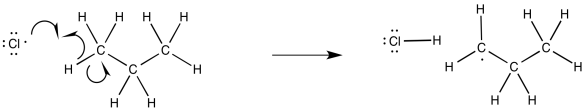
The newly-formed alkyl radical would be able to engage in propagation reactions as well. For example, it could react with another chlorine molecule. It would produce a new chlorine radical and the reaction product, chloroproane.
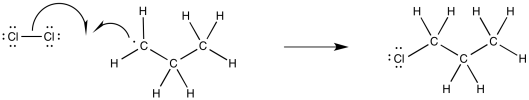
Alternatively, if we wanted to imagine the full range of elementary steps in radical reactions, we could picture a termination step, with another chlorine combining with that alkyl radical to form a chloroalkane product.
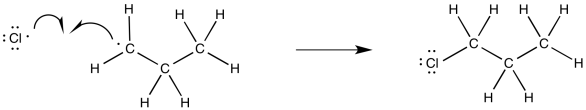
Propose a mechanism, with curved arrows, for the formation of 2-chloropropane via radical chlorination.
- Answer
-
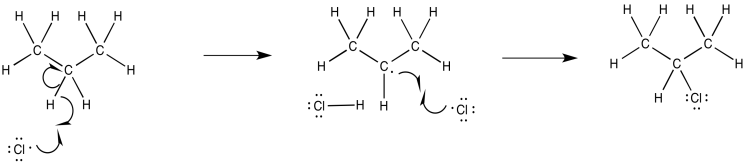
Propose a mechanism, with curved arrows, for the following variation on radical chlorination.
- Answer
-
Radical chlorination of pentane also results in multiple products. One of them is shown below. What other isomers are formed?
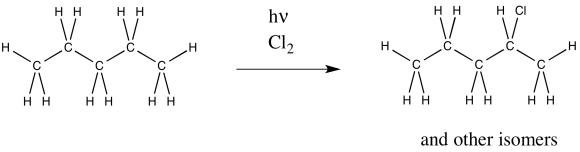
- Answer
-
2-chloropentane is shown; 1-chloropentane and 3-chloropentane would form, too.
Which product would be the major one in radical chlorination of propane? Why?
- Answer
-
2-bromopropane.
Clearly, radical halogenation could result in a mixture of products. That's because there are different hydrogen atoms that could be extracted in the first propagation step. Abstracting a hydrogen atom from the middle carbon of propane would lead ultimately to 2-chloropropane. Abstracting a hydrogen atom from either of the end carbons of propane would lead to formation of 1-chloropropane.


