6.3: Radical Initiation- Radical Stability
- Page ID
- 190040
Bond strength isn't just about the interaction of the two fragments bonded together. It is also influenced by the stability of those two species on their own. When the bond is broken, what pieces are left over?
The formation of radicals may be driven by the weakness of a particular bond. In terms of radical formation via bond homolysis, the reaction is more product-favored if the bond being broken is weak. In other words, the bond is not very low in energy, so the overall reaction may become more downhill (or at least less uphill). In that case, forward reaction is favored because of reactant destabilization.
However, a downhill reaction could also occur through product stabilization. For example, we have already seen that larger, more polarizable atoms form more stable radicals. Iodine radicals are more stable than bromine radicals, and sulfur radicals are more stable than oxygen radicals.
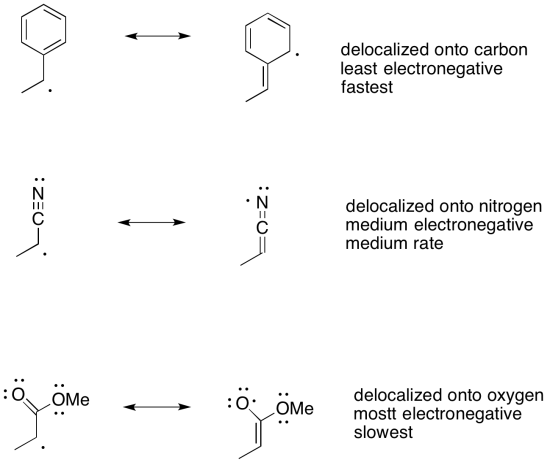
There are other factors, too. One of the most important factors is resonance. We have seen that the stability of anions and cations is strongly influenced by delocalization. Factors that spread the excess charge onto multiple atoms, rather than allowing charge to concentrate on one atom, make charged species much more stable.
For example, carbon-based anions are relatively unstable, but a delocalized carbanion is within the realm of possibility. Enolate ions are particularly easy to obtain because negative charge is partially delocalized onto a more electronegative oxygen atom. Delocalization also strongly stabilizes radicals. It is one of the most important factors in the stability of carbon-based radicals.
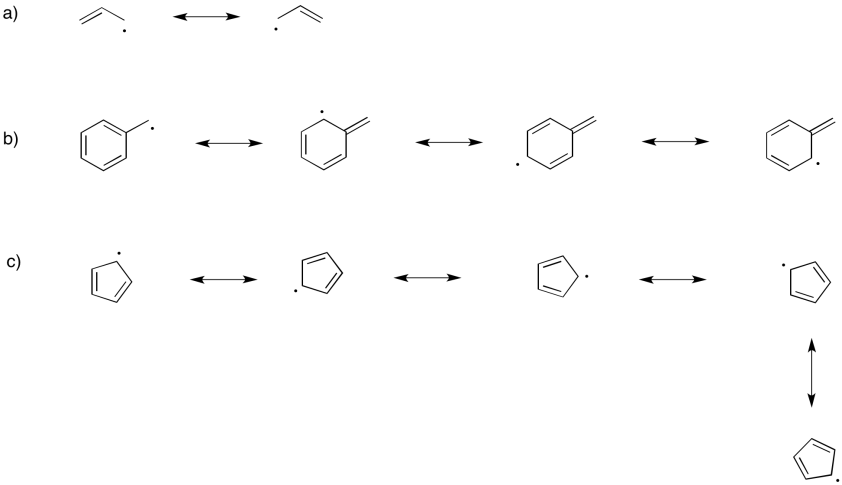
Illustrate the resonance stabilization in the following radicals
a) allyl, CH2CHCH2 b) benzyl, CH2C6H5 c) cyclopentadienyl, C5H5
- Answer
-
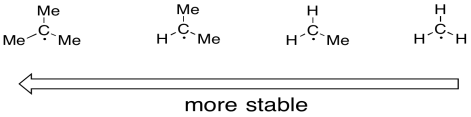
Radicals on carbon atoms are also stabilized when they are in more substituted positions. just as carbocations are more stable if they are on more substituted positions, carbon radicals are also more stable in these positions. A tertiary radical is more stable than a secondary one. A secondary radical is more stable than a primary one.
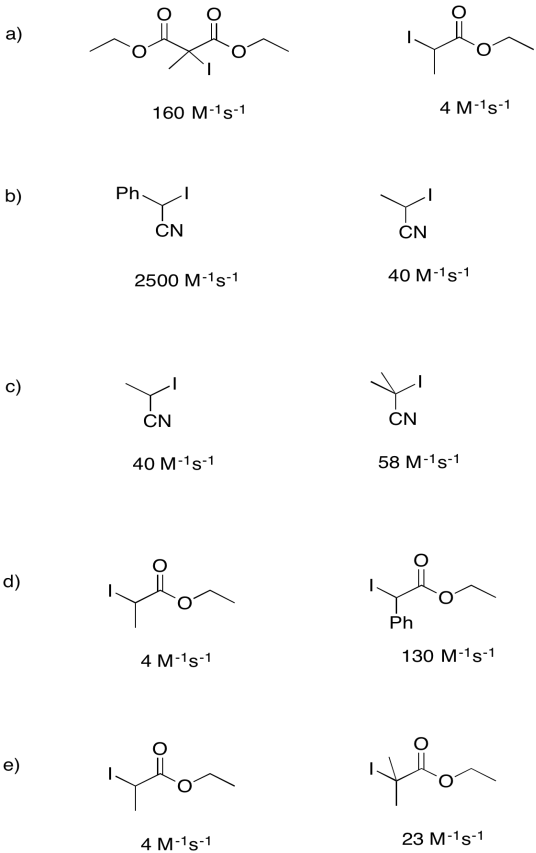
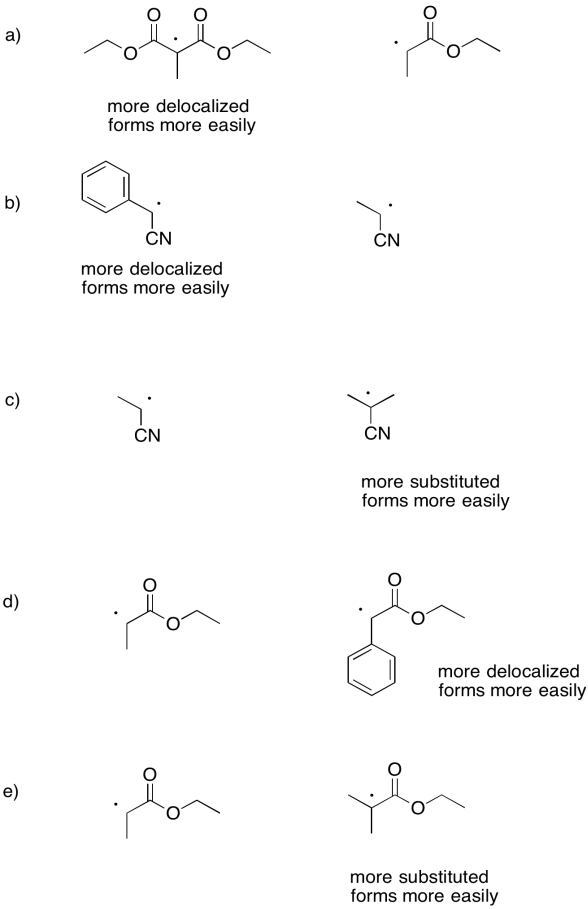
Rate constants for the dissociation of the following initiators to form an iodine atom and a radical were measured under a specific set of conditions. For each pair, explain why one compound undergoes homolysis more quickly.
- Answer
-
Predict the relative order of bond dissociation rates for the following initiators, which would each form a tellerium radical and a carbon radical.
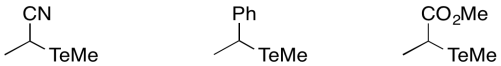
- Answer
-