4.16: Nucleophilic Substitution in Synthesis - Alcohols and Ethers
- Page ID
- 200811
Nucleophilic substitution reactions can be useful in organic synthesis. Mostly they are used for the interconversion of functional groups. For example, an alkyl halide might be transformed into an alcohol, or into an ether.
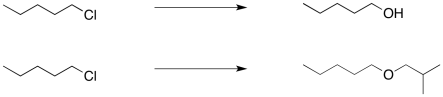
The trouble is, these seemingly simple steps can be very difficult. That's because the hydroxide needed to make an alcohol from an alkyl halide is really quite basic. So is the alkoxide that would be needed in order to turn an alkyl halide into an ether. Instead of substitution, you may get an elimination reaction.
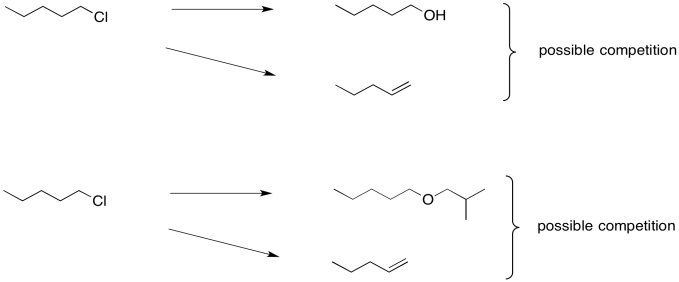
Who cares? Well, you would care if you were working on the synthesis of an antimalarial drug that could save millions, or an anticancer drug, or anything of that nature. Maybe that ether forms the last crucial part of the pharmacophore that will bind the drug to its target. Without this reaction, it may be thousands of times less effective. So this little reaction could be very important.
Clearly, the best thing to do would be to make sure a substitution reaction happened, and not that elimination. We need the reagent to be a nucleophile, not a base.
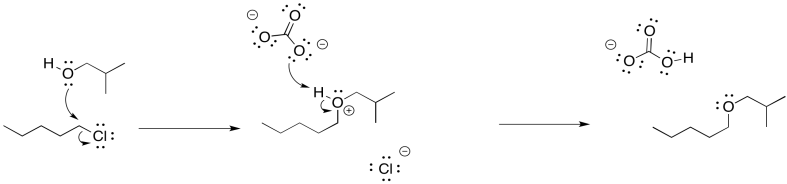
In the case of the alcohol synthesis, we could use water as the nucleophile, rather than hydroxide. Water is certainly less basic than hydroxide ion. It is still nucelophilic, though, because it still has a lone pair. If we add it to an alkyl chloride, the water will displace the chloride, and then the extra proton will be plucked off the positive oxygen atom by the chloride, leaving us with the alcohol.
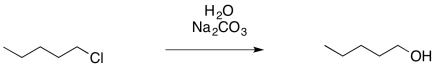
That last step would form hydrogen chloride, a corrosive acid, and that could cause problems. To counteract that possibility, we will want to add a weak base so that the HCl gets neutralized. Sodium carbonate (Na2CO3) or sodium bicarbonate (NaHCO3) may be good options, because they are mildly basic and they dissolve in water.
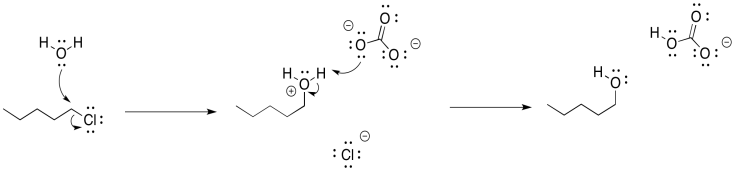
Alternatively, we could just use sodium hydroxide as the base. That gets us back to the original problem. However, in order to avoid elimination, we would use very dilute sodium hydroxide. We would keep its concentration low enough that the alkyl halide is much more likely to react with the water than with the hydroxide ion, for the simple reason that it is much more likely to run into a water molecule than a hydroxide ion. However, once that oxygen donor atom picked up a positive charge, it would be more attractive to the hydroxide ion, and the hydroxide would then come in for the proton.

In the same way, if we wanted to make an ether, we might use an alcohol as the nucleophile rather than the much more basic alkoxide ion. We would add a weak base to sponge up the extra proton and avoid formation of a strong acid.
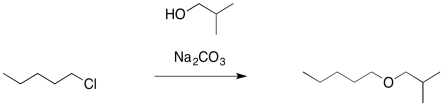
- A neutral nucleophile is less basic than an anionic one, and may avoid elimination reactions
- A weak base can be used to scavenge protons from the reaction
Provide a mechanism for the following reaction.

Answer-
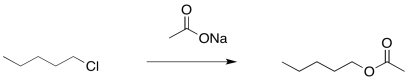
There is another approach to limiting the amount of elimination during a substitution step to form an alcohol. It also involves the use of a more stable nucleophile than a hydroxide ion. However, it employs a more reactive anionic nucleophile, rather than the neutral water. If an acetate ion is used instead, very little elimination usually occurs. An ester is formed as a product. There isn't much elimination because the acetate ion is resonance stabilised. More stable nucleophiles often undergo substitution rather than elimination.
Of course, we didn't want an ester; we wanted an alcohol. No problem. Esters can be saponified relatively easily -- that is, broken down into an alcohol and a carboxylate. Just add a hydroxide and water. Now the stronger carbonyl electrophile is a better target for the hydroxide and the reaction is pretty well assured to get to the right place.
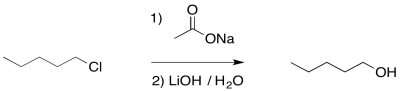
Overall, the reaction is actually a sequence of several events.
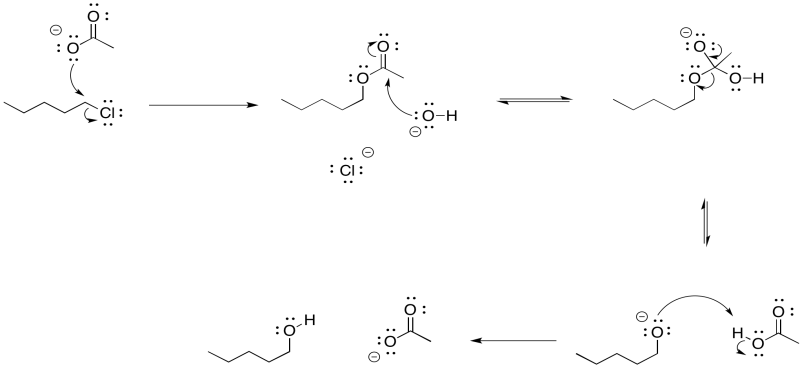
- Acetate esters are readily converted into alcohols under basic conditions
- By turning an alkyl halide into an ester first, and then into an alcohol, we can limit competition with elimination
We can't take the same approach in ether synthesis. An ether is an oxygen bridge between two tetrahedral or sp3 carbons; we can't have resonance stabilisation and still have those two sp3 carbons. Instead, another strategy is sometimes employed during addition of an anionic nucleophile to an alkyl halide. An alkoxide ion is still employed, but care is taken in how the alkoxide and the alkyl halide are chosen. Because the ether is symmetric -- it is two tetrahedral carbons attached to an oxygen -- either side could originate as the alkoxide and either side could originate as the alkyl halide.
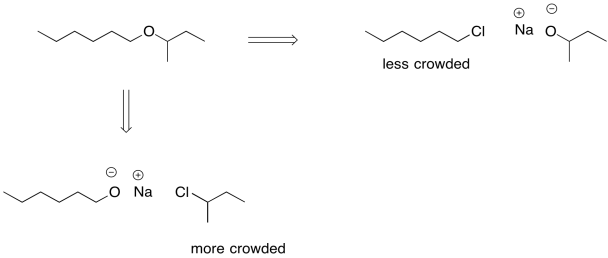
If the alkyl halide is chosen so that steric crowding is minimized, there is a lower chance of an accidental collision between the alkoxide and a beta hydrogen on that alkyl halide. In some cases, we might even be able to choose the alkyl halide so that elimination is not possible at all. If possible, we can use an alkyl halide that doesn't have any beta hydrogens.

In general, the use of alkoxide ions as nucleophiles can be pretty successful if done carefully, and this approach to making ethers even has its own name. It's called the Williamson ether synthesis.
- In the Williamson ether synthesis, the less crowded half of the ether is formed from the alkyl halide
- In some cases, an alkyl halide may also be chosen because elimination is not physically possible with that structure
Propose Williamson ether syntheses of the following compounds.
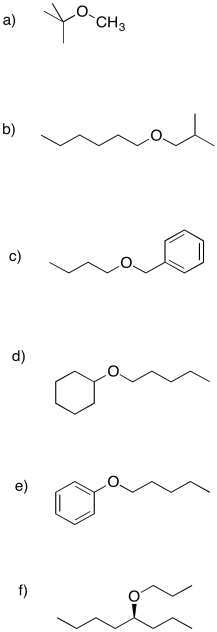
Answer-
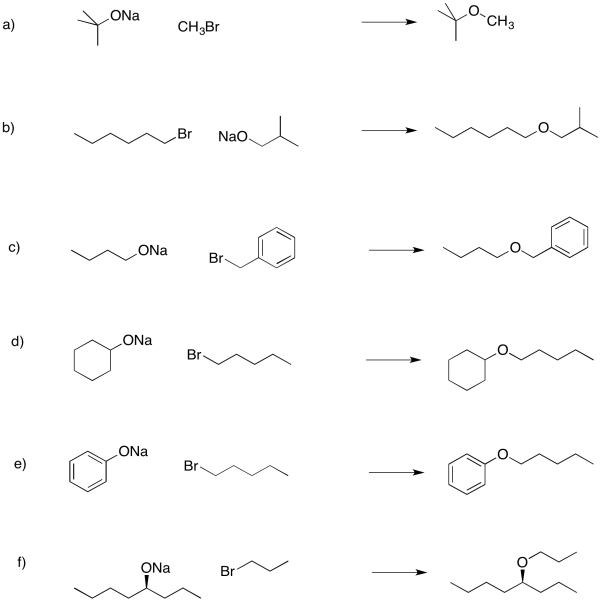
Provide products of the following reactions.
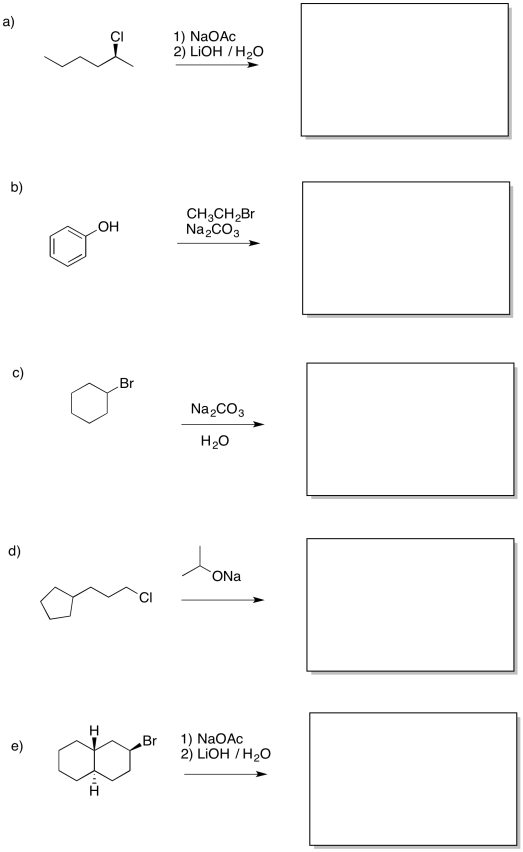
Answer-



