4.15: Factors Influencing the Elimination Mechanism
- Page ID
- 200810
The factors that influence whether an elimination reaction proceeds through an E1 or E2 reaction are almost exactly the same as the factors that influence the SN1/SN2 pathway. Cation stability, solvents and basicity play prominent roles. However, basicity may be the single most important of these factors.
By analogy with substitution reaction, in which elimination mechanism does cation stability play a strong role: E1 or E2?
- Answer
-
Cation stability is important in an E1 reaction.
Draw an example of an alkyl halide that is likely to undergo an E1 elimination.
- Answer
-
Any tertiary alkyl halide would be a good example. Benzylic alkyl halides would also be good examples if they are either secondary or tertiary.
By analogy with substitution reactions, what mechanism would be promoted by protic solvents: E1 or E2?
- Answer
-
Protic solvents could promote E1 reactions.
Basicity refers to the strength of the base. Which mechanism is more likely to occur with strong bases: E1 or E2?
- Answer
-
A strong base could promote an E2 reaction.
Because strong bases can be thought of as very reactive nucleophiles, it might not be surprising that they tilt the likely reaction towards E2. These compounds are so reactive that they are much more likely to abstract a proton than be guided to the electrophilic carbon. They are also sufficiently reactive that they can intercept the electrophile on a timescale faster than it takes for the electrophile to ionize. Even under conditions that might otherwise be expected to lead to an SN1 or E1 reaction, if the base is strong, E2 will frequently prevail.
Of course, that general rule is subject to limitations. If there is no beta-hydrogen to abstract, then there will be no elimination of any kind. The compound will be limited to substitution reactions. Furthermore, if the C-H bond cannot line up with the C-LGp bond in such a way as to allow them to break together, forming a pi bond at the same time, then an E2 mechanism would be impossible. However, the lack of possibility for an antiperiplanar relationship is not that common. If we are dealing with an alkyl chain, rotations around sigma bonds will be sufficient to bring the C-H and C-LGp bonds into alignment. Only in cyclic structures, in which rotation is much more restricted, would there be a possibility that an antiperiplanar relationship would be completely prevented.
So we need to keep E2 in mind when we are dealing with strong bases. Those compounds are generally unstabilized oxygen anions: hydroxide and alkoxides (such as CH3O- or CH3CH2O-). Very strong bases would also fall into this category. These bases are generally unstabilized carbon and nitrogen anions or semianions (such as amide, NH2-, or alkyllithiums such as CH3Li or CH3CH2CH2CH2Li). Bases that are stabilized in some way, such as resonance stabilized carboxylates (like CH3CO2-) or enolates (like CH3COCH2-) are much weaker, and are much more likely to act as nucleophiles than as bases.
E1 reactions, like SN1 reactions, are really only possible when cation stability allows them to occur. Remember that cation formation is the slow step in these mechanisms. If the cation is too unstable to form in the first place, then there is no going down this road. That means tertiary alkyl halides are good candidates for E1, but primary alkyl halides are not. Under the right conditions, such as in the presence of a protic solvent, secondary cations might also be coaxed into being.
Whether at that point the cation undergoes an SN1 or an E1 reaction depends less on the nucleophile than you might expect. At this point we are dealing with more or less weak bases; a strong base would have carried out an E2 while it had the chance. Instead, the two possibilities may compete, giving mixtures of products, but temperature is often the major factor determining which way the reaction will go. At higher temperature, E1 reactions are favored for entropic considerations. At lower temperature, SN1 may dominate.
Sometimes, E1 reactions can be carried out in the presence of a strong base, but the base is very dilute. As a result, the base is not as likely to encounter the alkyl halide until the alkyl halide has already had time to dissociate into anion and cation. At that point, the base just comes in and deprotonates the cation.
Predict the major products of the following reactions, and the mechanism type (SN1, SN2, E1, E2) by which the products are formed.
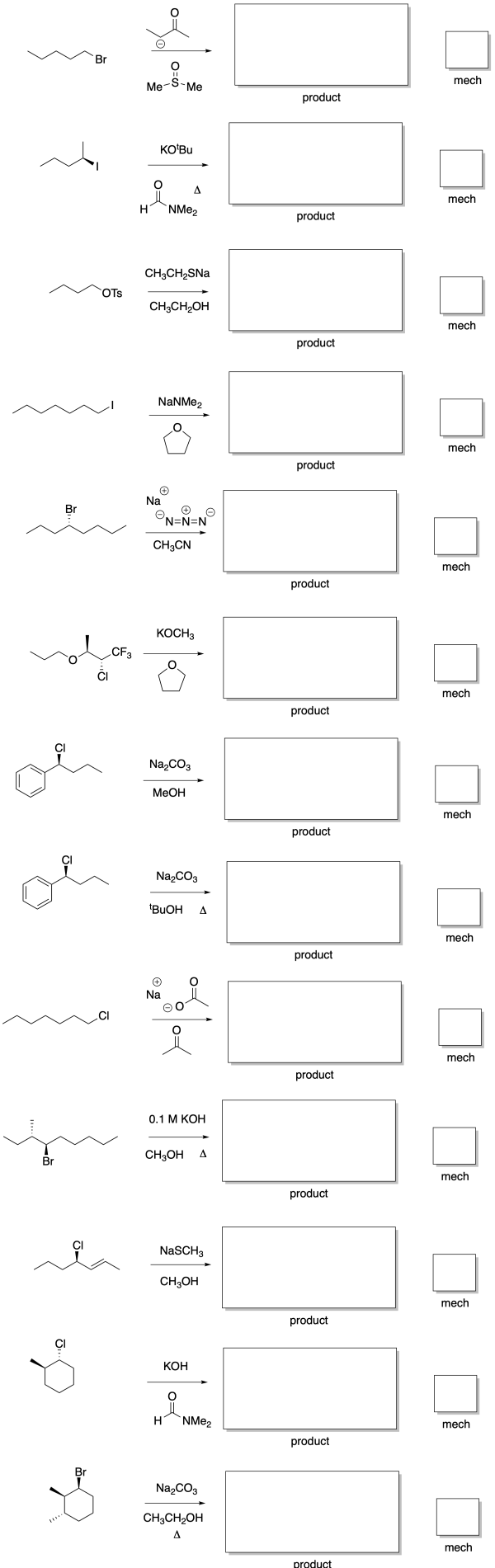
- Answer
-