3.12: Organic Oxidation
- Page ID
- 195895
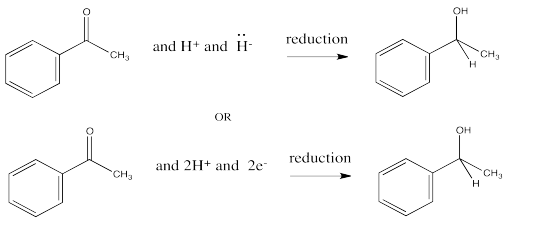
You may recall that conversion of an aldehyde or ketone to an alcohol is referred to as a reduction. The hydride from an NADH molecule or a BH4- anion acts as a nucleophile, adding H- to the carbonyl carbon. A proton source can then protonate the oxygen of the resulting alkoxide ion, forming an alcohol.
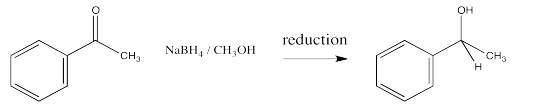
In this reduction, two electrons and two protons are donated to the carbonyl compound to produce an alcohol.
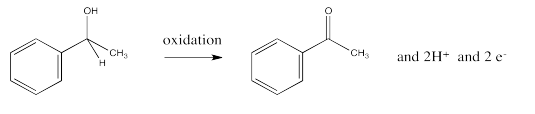
The opposite process, the loss of two protons and two electrons from an alcohol to form a ketone or aldehyde, is an oxidation.
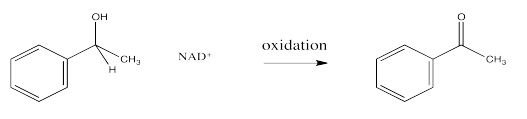
In biological pathways, oxidation is often the microscopic reverse of reduction. That means that the products of a reduction, NAD+ and an alcohol, could react together under the right circumstances to form NADH and a carbonyl. The reduction of NAD+ by a hydride donor is possible because, although the NAD+ loses the aromaticity of its nicotinamide ring upon becoming NADH, it also loses its positive charge. Charge stabilization is frequently an energetic problem for molecules.
This general type of reaction, hydride transfer reduction, has been adapted by Ryoji Noyori, of Nagoya University in Japan, to produce a single enantiomer of a chiral alcohol product. Noyori's work on this reaction, and others, led to him being awarded the Nobel Prize in Chemistry in 2001.
Exercise \(\PageIndex{1}\)
Show the two enantiomers that could be produced from reduction of acetophenone, CH3(CO)C6H5
Exercise \(\PageIndex{2}\)
Provide a mechanism with arrows for the Oppenauer oxidation of benzyl alcohol, C6H5CH2OH.
Exercise \(\PageIndex{3}\)
Explain why acetone is used as the solvent in an Oppenauer oxidation.
- Answer
-
Add texts here. Do not delete this text first.
Exercise \(\PageIndex{4}\)
Meerwein-Ponndorf-Verley reduction of a ketone is carried out with aluminum tris(isopropoxide) in isopropanol as solvent. Provide a mechanism for the reduction of acetophenone, CH3(CO)C6H5, via this reaction.
Exercise \(\PageIndex{5}\)
Explain why isopropanol is used as the solvent in a Meerwein-Pondorf-Verley reduction
A second general method for alcohol oxidation employs a "redox-active" transition metal to accept a pair of electrons from from an alcohol during the oxidation. Because oxidation of an alcohol formally involves the loss of two electrons and two protons, a proton acceptor is also involved in this oxidation. There are many redox-active metals, but one of the most commonly used is Cr(VI). When Cr(VI) accepts a pair of electrons, it becomes Cr(IV).
In order to look at how chromium oxidation works, we'll use chromium oxide, CrO3, as an oxidant and water as a solvent. Note that water could also act as a proton acceptor or proton shuttle, moving protons from one place to another as needed. To carry out an oxidation, a number of events need to happen.
- The alcohol needs to bind to the chromium.
- A proton needs to be removed. This event is helped by the formal positive charge on the alcohol after it donates a lone pair to the chromium.
- A second proton must be removed and a pair of electrons given to the chromium for good.
In reality, CrO3 isn't used that often as an oxidant. It tends to catch fire when mixed with organic compounds. Instead, a variety of other chromium compounds are used.
Exercise \(\PageIndex{6}\)
In determining an oxidation state, we imagine giving both electrons in a bond to the more electronegative atom and looking at the resulting charges on the ions that result. Assuming all of the oxygens in chromium oxide can be thought of as dianions, confirm that the chromium can be thought of as a Cr6+ cation (in other words, in oxidation state Cr(VI)).
- Answer
-

Exercise \(\PageIndex{7}\)
By the reasoning used in the previous question, determine the oxidation state of the transition metal in the following compounds. Note that in some cases, there is an anion and cation in the compound.
a) KMnO4 b) NaIO4 c) Ag2O d) OsO4 e) (CH3CH2CH2)4N RuO4
- Answer
-
Add texts here. Do not delete this text first.


