2.6: Hapticity
- Page ID
- 189930
When a lone pair is bonded to a transition metal, then the atom with the lone pair is forming a bond to the metal. When a pi bond is coordinated, which atom is bonded to the metal? Both of them. Three atoms are involved in this bonding situation, instead of just two. Both carbons that form the original pi bond are now donating that pi bond to the metal.
When ligands are bound to a metal via a conjugated pi-system, describing the mode of binding might seem even trickier. If there are two double bonds in a row, then all four of the atoms that form those two pi bonds are donating to the metal. Furthermore, because the bond is conjugated, we can think of this as one long pi bond. The bond from the pi bond to the metal involves all four donor atoms, plus the metal atom.
Contrast that situation with two separate pi bonds that are not conjugated. If a ligand contains two separate pi bonds, it is a bidentate donor. Bidentate ligands bind through two donor sites. We think of a ligand like 1,2-ethanediamine as binding through the lone pairs on both nitrogen atoms. We would think of 1,5-hexadiene as binding through the pi bond at either end of the chain. However, 1,3-butadiene is a little different, because of the participation of all four carbons in bonding to the metal through one conjugated bond.
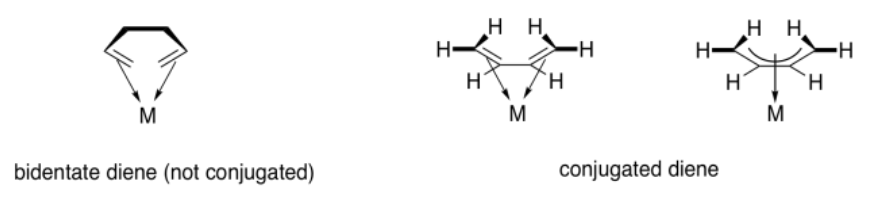
The term used to describe the participation of multiple atoms simultaneously during pi coordination is hapticity. A regular alkene, like ethene or propene, is a dihaptic donor; two carbons participate in donation of one bond to the metal. A conjugated alkene, like 1,3-butadiene, is a tetrahaptic donor. Four carbons participate in donation of a conjugated pi bond to the metal. Of course, this conjugated diene can donate four electrons at once, forming something a little like a double bond to the metal.
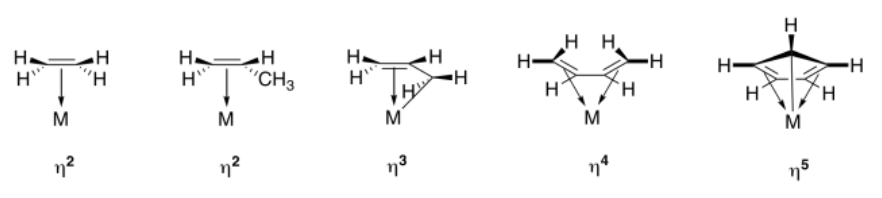
In the drawings above, the symbols, η2 or η3, etc. (read "eta-two" or "eta-three") refer to the hapticity of the ligand. An η2 ligand is dihaptic, with two atoms sharing in the donation from the pi system; an η3 ligand is trihaptic, with three atoms sharing in the donation from the conjugated pi system.
The following alkenes form complexes with silver. Describe their probable mode of binding as η2, etc.:
- CH2CHCHCH2
- CH2CHCH2CHCH2
- CH2CHCH2CH2CHCH2
- CH2CHCHCHCHCH2
- Answer a
-
There are two double bonds here, and they are conjugated: CH2=CH-CH=CH2. The conjugated double bond would allow the ligand to bind η4.
- Answer b
-
There are two double bonds here, but they are not conjugated: CH2=CH-CH2-CH=CH2. Each double bond would bind η2, and the ligand would be able to bind in a bidentate fashion, but since the double bonds are not conjugated and binding all in a row, we would not describe binding as η4. It would most commonly be described as η2,η2; that simply means each double bond is an η2-donor, and there are two of them. It could also be considered a κ2 donor because of its denticity.
- Answer c
-
This is another non-conjugated case: CH2=CH-CH2-CH2-CH=CH2. In terms of hapticity, it could be described as η2,η2.
- Answer d
-
There are three double bonds here, and they are conjugated: CH2=CH-CH=CH-CH=CH2. The conjugated double bond would allow the ligand to bind η6.
Cyclic, conjugated systems make good ligands for transition metals. In each of the following cases,
- describe the hapticity.
- indicate the number of electrons donated to the metal.
- indicate the charge on the ligand.

- Answer a
-
The ligand is bound η5; it donates 6 electrons, from two double bonds and one lone pair; the ligand has a charge of -1.
- Answer b
-
The ligand is bound η4; it donates 4 electrons, from two double bonds; the ligand has no charge.
- Answer c
-
The ligand is bound η7; it donates 8 electrons, from three double bonds and one lone pair; the ligand has a charge of -1.
- Answer d
-
The ligand is bound η6; it donates 6 electrons, from three double bonds; the ligand has no charge.
Sometimes, conjugated ligands might "slip", donating fewer than the maximum number of electrons to the metal. In the following cases, indicate:
i) the hapticity shown in the picture.
ii) the maximum hapticity possible with the ligand.
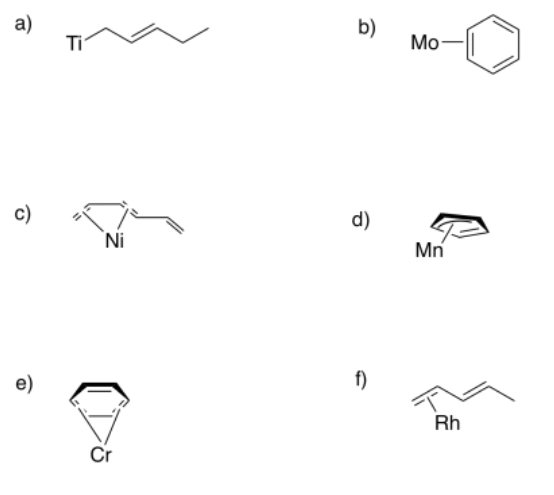
- Answer a
-
The ligand is bound η1; it donates one lone pair; however, it could donate an additional pi bond and then it would bind η3.
- Answer b
-
The ligand is bound η2; it donates one pi bond; however, it could donate two more pi bonds and then it would bind η6.
- Answer c
-
The ligand is bound η4; it donates two pi bonds; however, it could donate one more pi bond and then it would bind η6.
- Answer d
-
The ligand is bound η3; it donates one lone pair and one pi bond; however, it could donate an additional pi bond and then it would bind η5.
- Answer e
-
The ligand is bound η4; it donates two pi bonds; however, it could donate one more pi bond and then it would bind η6.
- Answer f
-
The ligand is bound η2; it donates one pi bond; however, it could donate one more pi bond and then it would bind η4.
One of the most common multidentate ligands is the cyclopentadienyl anion, often abbreviated Cp.

- CpH is easily deprotonated to form Cp-. Explain why.
- How many electrons does Cp donate to a metal?
- The archetypal Cp complex is ferrocene, Cp2Fe, the structure of which was determined by Geoff Wilkinson, in work that led to him being awarded the Nobel Prize in 1973. Draw the structure of ferrocene.
- Count the electrons on the iron in ferrocene.
- Answer a
-
The resulting anion has aromatic stability. It is cyclic, fully conjugated, flat and has an odd number of electron pairs.
- Answer b
-
Cp anion could bind to a metal through just one pair or through two pairs, but in most cases it will bind via three pairs of electrons.
- Answer d
-
Valence count on metal: 8
Count on metal, correcting for +2 charge: 6
Donated from ligands: 2 x 6 = 12
Total: 18
Predict the most probable binding modes of the following ligands (monodentate, trihaptic, etc.).
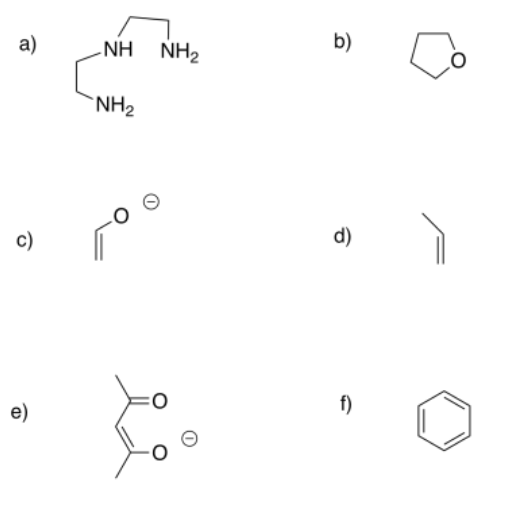
- Answer
-
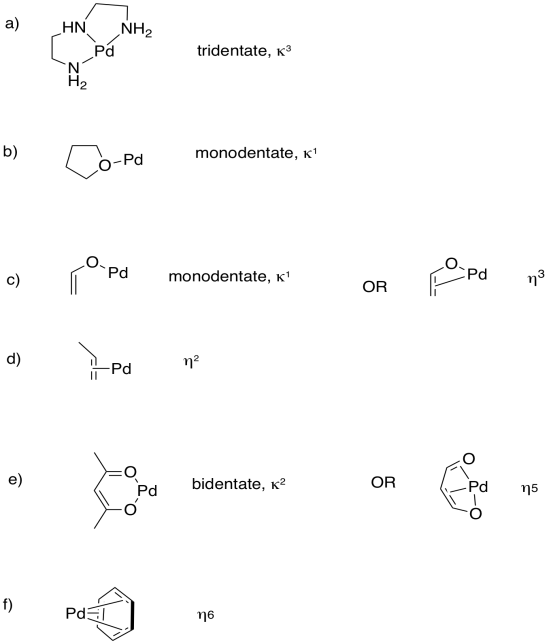
Using ideas from denticity, explain the differences seen in the equilibrium constants for the formation of silver(I) complexes of the following alkenes:
- CH2=CHCH=CH2; K = 4.2
- CH2=CHCH2CH=CH2; K = 10.2
- CH2=CHCH2CH2CH=CH2; K = 28.8
- Answer
-
This problem deals with the "bite angle" of the ligand. Remember, a chain of atoms becomes more flexible the longer it gets, because of the possibility for rotation around each bond along the chain. As the two double bonds move further apart from each other (one bond apart in (a), two bonds apart in (b) and three bonds apart in (c), the chain can "open up" and bind with a more optimal overlap with the metal.


