1.5: Reversibility and Le Chatelier
- Page ID
- 189922
Sometimes, there is not a big difference in energy between reactants and products of a reaction. What happens then? Does the reaction go forward, because it will not cost a lot of energy? Or does it not proceed, because there isn't enough driving force?
For example, one simple reaction that occurs all the time is the reaction of water with carbon dioxide. This is a reaction that happens when carbon dioxide dissolves in lakes, rivers and oceans. It even happens in your own bloodstream.

Water reacts with carbon dioxide to form carbonic acid.
However, carbonic acid also decomposes spontaneously in water. It reacts to form carbon dioxide and water.
In other words, this is a reaction that can go either direction. It can go forwards or backwards. It is an example of an equilibrium reaction. An equilibrium reaction is one that is energetically balanced, so that it really isn't favored to go in either direction.
Equilibrium reactions are extremely important in nature, partly because of the forward and reverse capabilities that they offer. In essence, they are reactions with an "undo" button. The reaction can proceed in one direction when needed, and it can proceed in the other direction when needed.
However, there are some inherent limitations involved. Frequently, equilibrium reactions only proceed "partway". That is, a group of molecules will start to produce products. However, at some point those products will begin reverting to the starting materials again. Eventually the system will settle out as a mixture of reactants and products.
What if it's really important that we have the products of the reaction at one point, with none of the reactants? And if later on we need the reactants, but not any of the products? It would be useful if there were a way to control the direction of an equilibrium reaction, so that we could "push" it to one side or the other.
Control of equilibrium reactions can be remarkably simple. It follows a rule that was observed by Henri le Chatelier (ah-REE luh shah-tell-YAY), a French industrial chemist, around 1900. Le Chaletelier noticed that equilibrium reactions often shift direction if the conditions of the reaction are changed.
In general, adding any product of the reaction shifts the balance back toward the reactants. If any product of the reaction is added, the reaction makes more starting materials. Thus, adding more carbonic acid to a carbon dioxide - water - carbonic acid mixture would result in reverse reaction, producing more water and carbon dioxide. Adding more carbon dioxide, on the other hand, would lead to production of more carbonic acid.
Here is a cartoon illustration of "le Chatelier's Principle" at work. Suppose red squares and blue ovals can react together to make black circles and green circles. Maybe there is a natural equilibrium in this reaction, so that the two piles of shapes are roughly equal in size.
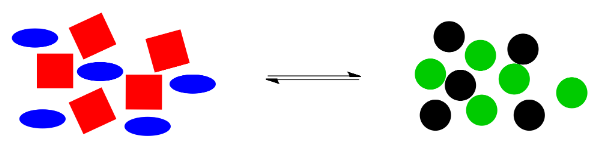
What would happen if something knocked this system off balance? For example, maybe black circles are highly elusive, and they just wander away as soon as they are formed. The system won't be in equilibrium anymore, because without those black circles, the balance will be upset, with not enough things on the right side for the number of things on the left.
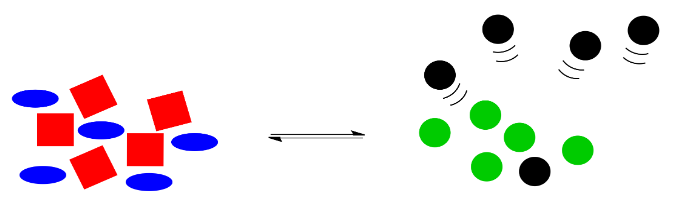
Le Chatelier noticed that nature automatically corrects for such changes. If some of the black circles disappear, the reaction will kick into action again, using up some red squares and blue ellipses to produce more green and black circles. The exact numbers of shapes won't return to exactly the same as before, because some of the black circles have still gone missing, but the system will have shifted to use up more reactants on the left and to produce more products on the right, so that the overall ratio between right and left is restored.
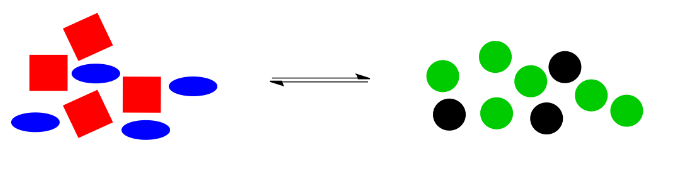
Alternatively, maybe we found a way to make the black circles stay where they are. Instead, we have dumped in a bunch of extra blue ellipses. Once again, the system is knocked off balance. This time, there is too much stuff on the left, compared to the amount on the right side.
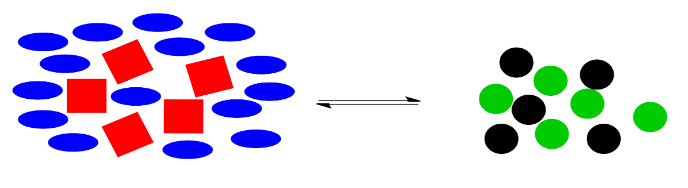
The reaction goes into action again. It uses up some of those extra blue ellipses (and, at the same time, some of the red squares) to produce more black and green circles, bringing the system back to the original ratio of right side shapes to left side shapes.
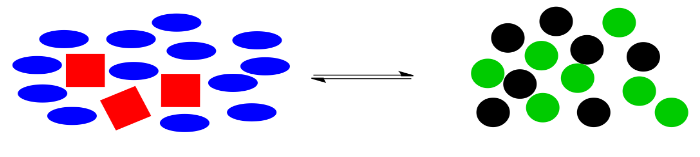
In general, if molecules are added to a system, the reaction will shift to bring the system back into equilibrium. If molecules are removed from the system, the reaction will also shift to bring the system back into equilibrium.
Furthermore, because heat can be consumed by (or produced by) reactions, temperature can sometimes be used to shift equilibria. If a reaction is exothermic, heat is a product of the reaction. Adding more heat will result in the reaction shifting to produce more reactants. Cooling the reaction (removing heat) would do the opposite: the reaction would shift to produce more heat, and more products.
In the cartoon, we have a shape-shifting reaction again, but this time the reaction releases energy (those are orange flames, symbolic of the heat produced).
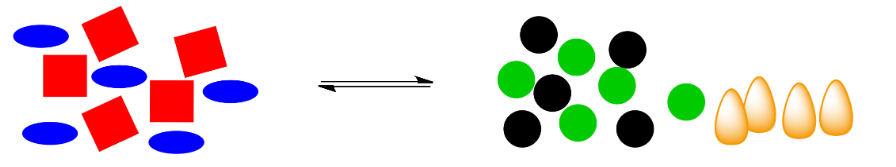
What happens if that energy is removed? For example, if heat is removed through addition of a pale blue ice cube, what will be the effect on the system?
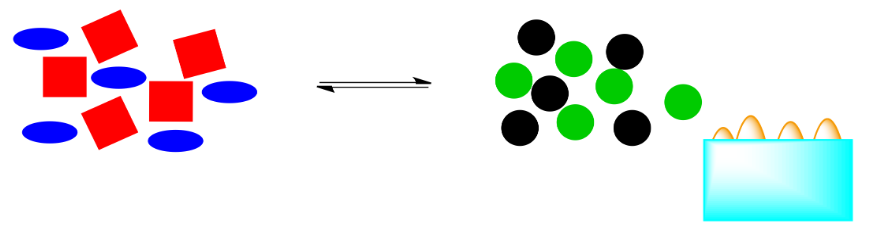
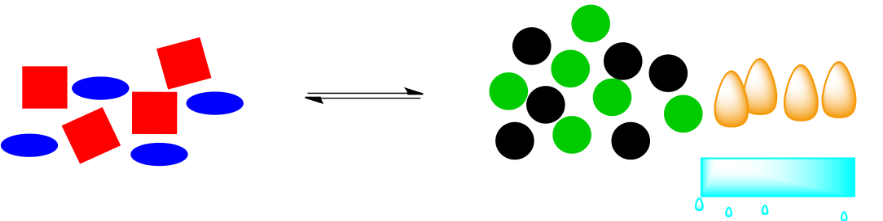
Those orange energy shapes (the "flames") were a part of the system. If they are removed, the system will have to shift in order to restore them. If the reaction pushes to the right again, more energy will be released, bringing the system back into equilibrium.
The water-gas shift reaction involves the production of hydrogen gas from steam and carbon monoxide. It is important both for the commercial production of hydrogen gas and for its application in fuel cells. At 300 K, the reaction (and an approximate energy produced) is shown below:
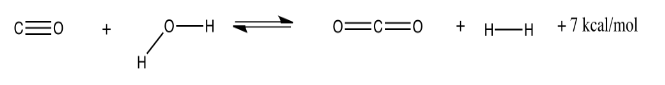
Explain what would happen if this gas-phase reaction is already at equilibrium and the following changes take place:
- The pressure of steam injected into the reaction is doubled.
- The temperature is raised to 450 K.
- The CO2 produced is "captured" and removed as carbonate.
- The temperature is lowered to 250 K.
- The pressure of CO added is cut in half.
- Answer
- Answer
-
The removal of any item produced on the right side of the reaction will shift the reaction to the right in order to restore equilibrium. On the other hand, adding any more of any of the items on the right will shift the reaction to the left.
Items on the left side will work in the opposite way. Adding more of anything on the left will shift the reaction to the right, to use up the newly added materials. Removing anything from the left will shift the reaction further left, to replace the items that were removed.
- Answer a
-
The amount of water increases, moving the reaction to the right. More products are made.
- Answer b
-
The amount of energy increases, moving the reaction to the left. Fewer products are made.
- Answer c
-
The amount of carbon dioxide decreases, shifting the reaction to the right. More products are made.
- Answer d
-
The amount of energy decreases, shifting the reaction to the right. More products are made.
- Answer e
-
The amount of carbon monoxide decreases, shifting the reaction to the left. Fewer products are made.
Hydrochlorination of acetylene (ethyne) is another gas-phase reaction. It is used to produce vinyl chloride, the starting material for the polyvinyl chloride commonly used to make the pipes in household plumbing. At 300 K, the reaction (and an approximate energy produced) is shown below:
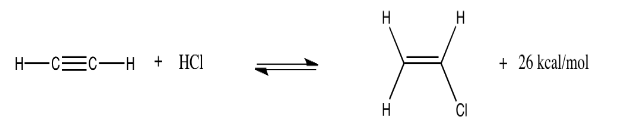
Explain what would happen if the system is at equilibrium and the following changes take place:
- The temperature is raised to 350 K.
- The pressure of HCl is doubled.
- The pressure of acetylene is cut in half.
- The temperature is dropped to 250 K.
- The overall pressure in the system is increased from one atmosphere to two atmospheres.
- Answer
- Answer a
-
The amount of energy increases, moving the reaction to the left. Fewer products are made.
- Answer b
-
The amount of hydrogen chloride increases, shifting the reaction to the right. More products are made.
- Answer c
-
The amount of acetylene decreases, shifting the reaction to the left. Fewer products are made.
- Answer d
-
The amount of energy decreases, shifting the reaction to the right. More products are made.
- Answer e
-
This question doesn't follow the pattern. However, because the products and reactants are all gases, we can think about the effect they would have on pressure if the reaction moved one way or the other. Because fewer gas molecules are produced on the right than the left, pressure would decrease on going from left to right (and increase on going from right to left). Thus, we can pencil in "pressure" as an item on the left side of the reaction. That means increasing pressure will shift the reaction to the right, making more products.
Production of ATP in the cell proceeds according to the reaction below, with an approximate energy indicated at 310 K.
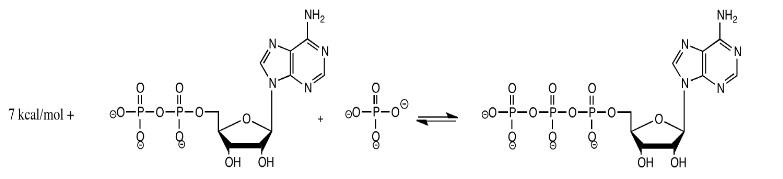
If the system is already at equilibrium, explain what happens when the following changes take place:
- The temperature is raised to 320 K (It's OK. This organism is really hardy and it can handle the temperature change).
- The temperature is lowered to 300 K.
- The supply of inorganic phosphate is doubled.
- Answer
- Answer a
-
The amount of energy increases, shifting the reaction to the right. More products are made.
- Answer b
-
The amount of energy decreases, moving the reaction to the left. Fewer products are made.
- Answer c
-
The amount of phosphate increases, shifting the reaction to the right. More products are made.
Nitric acid, HNO3, is a common industrial chemical. For example, it is used to make azo dyes that are employed in paints. Nitric acid production involves the following reaction, with an approximate energy change indicated at 300 K.
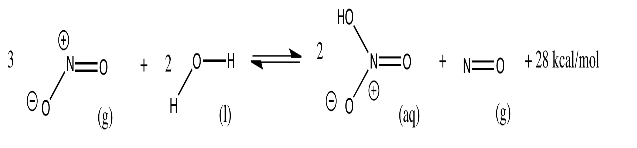
Note that this is a multi-phase reaction: it involves gases (g), liquids (l) and aqueous solutions (aq, something dissolved in water). Explain what would happen if production were run under the following conditions:
- The NO2 gas is introduced into a chamber that contains a tank of water. After reacting for a while, the gas is released and the water, containing the aqueous solution of nitric acid, is drained from the tank.
- The NO2 gas is introduced into a chamber that contains a tank of water. Periodically, the water is drained from the tank, and new water is introduced, without releasing any gases.
- The NO2 gas is continually introduced into a chamber, and there is a vent that slowly releases gases from the chamber at all times. There is also a constant flow of water into and out of the chamber.
- Answer
- Answer a
-
The nitric acid would build up in the water, and the NO gas would build up, until equilibrium is reached. The nitric acid in the water would be limited by that equilibrium point.
- Answer b
-
Periodically removing the nitric acid solution and adding fresh water would help to shift the reaction further to the right, although the eventual buildup of NO gas might prevent the reaction from shifting too far.
- Answer c
-
A constant source of both water and nitrogen dioxide (nitric oxide) would help to push the reaction to the right. Although allowing gases to vent would limit the amount of nitrogen dioxide in the system, it would also prevent a buildup of nitrogen monoxide (nitrous oxide), which would otherwise push the reaction to the left, eventually.


