7.15: Instrumentation Using Chromatography
- Page ID
- 195395
Liquid chromatography is often done with more sophisticated equipment. One kind of method is called "high performance liquid chromatography" or HPLC. Rather than packing stationary phase into a glass column, a steel column containing the stationary phase can be purchased. The column can be plumbed into a system that contains a solvent pump to push eluent through the column. After passing through the column, the liquid may go into a UV spectrometer so you can detect when compounds are eluting from the column. The whole apparatus is controlled by a computer. By clicking a button, you can change how quickly the solvent flows. You can easily change the ratio of solvents in the eluent by clicking a button, too.
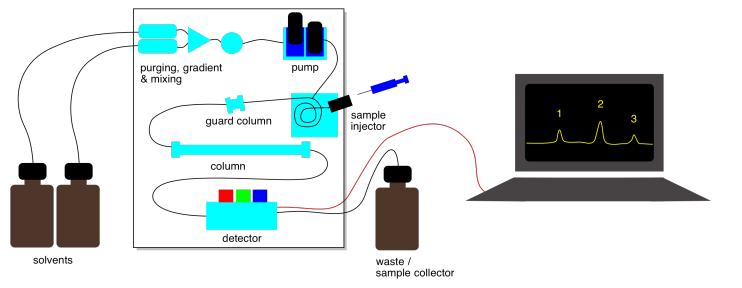
In addition to a UV spectrometer, other instruments can be used with an HPLC system to get information about compounds being eluted . One of the most important is mass spectrometry (MS). Liquid chromatography-mass spectrometry (LC-MS) can be used to determine the molecular weights of the compounds as they elute. That information can be used to help identify the compound.
Gas chromatography is another important variation that you should know about. Instead of passing a liquid over the stationary phase, an inert gas moves over the stationary phase. The inert gas may be helium or nitrogen. The equilibrium here is between compounds absorbed onto the stationary phase and compounds moving in the gas phase. Intermolecular attractions with the stationary phase play a role in GC, but so does the boiling point of the compounds.
Because most compounds are not very volatile, they would spend all their time sitting on the solid phase under normal conditions. For that reason, the column in a gas chromatograph is placed inside an oven. The temperature in this oven is carefully controlled so that compounds will spend a greater fraction of time in the gas phase. A heating element in the oven can increase the temperature, and a fan is present to help cool it down when.
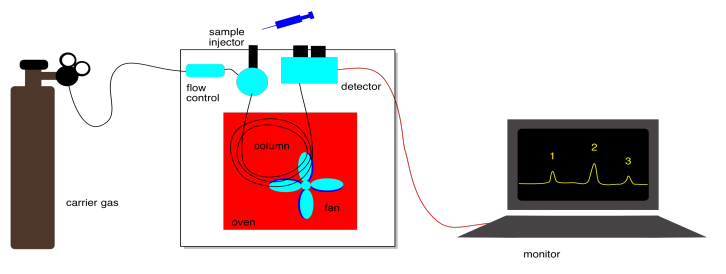
The eluent can't be varied in GC. It is just an inert gas. To control separation of compounds in GC, we can change the pressure of the inert gas, which controls how quickly the gas flows. We can also control the temperature, which influences how much time compounds spend moving along in the gas phase . We can also choose different kinds of columns with different stationary phases.


