20.3: The Building Blocks of Biochemistry
- Page ID
- 49602
It has been estimated that even a unicellular organism may contain as many as 5000 different substances, and the human body probably has well over 5 000 000. Only a few of these are exactly the same in both species, and so the total number of different compounds in the living portion of the earth (the biosphere) is approximately (105 compounds/species) × 106 species = 1011 compounds. If it were possible for chemists to synthesize one of these every second, 24 hours a day, 7 days a week, about 3000 years would be required to make all of them—obviously a hopeless task, even if we knew the composition and structure of each one.
How then can we make sense out of the chemistry of living systems? Fortunately nearly all the substances found in living cells are polymeric—they are built up by different combinations of a limited number of relatively small molecules. For example, the basic structures of all proteins in all organisms consist of covalently linked chains containing 100 or more amino acid residues. Only 20 different amino acids are commonly incorporated in proteins, but the number of ways of arranging 100 of these in a chain taking any of the amino acids at random for each place in the chain is 20100 \(\cong\) 10130, allowing an almost infinite variety of structures. Just as an understanding of the properties of atoms and their bonding characteristics was a significant aid in predicting the chemistry of molecules, a knowledge of the properties of simple molecular building blocks gives us a starting point for the study of biochemistry. Each of the building blocks and their polymeric forms has at least one major role to play in the chemistry of life. Most are quite versatile, serving several functions. Let us take a look at the building blocks of biochemistry:
Sugars, or carbohydrates are molecules that follow the form Cx(H2O)y. A simple sugar can serve as an energy source for an organism. Simple sugars can dimerize into disaccharides or polymerize into Polysaccharides. Uses for polysacharides range from energy storage, such as glycogen stored in your liver and muscles, to structural support, such as cellulose that makes up cell walls for plants.
 |
||
| glucose, a monosaccharide | sucrose, a disaccharide | glycogen a polysaccharide |
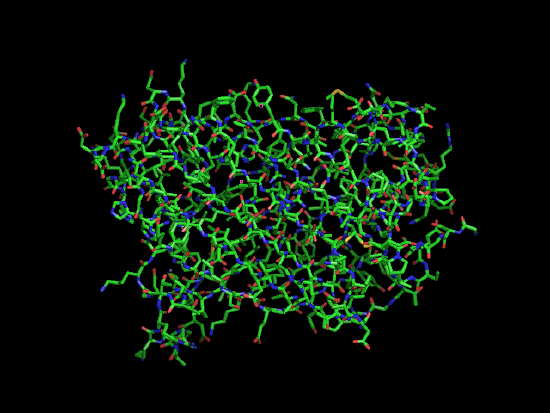
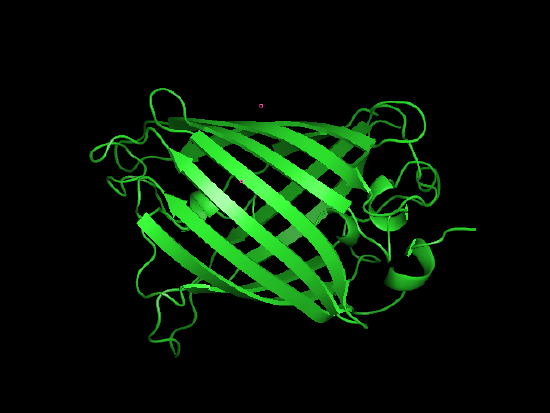
Amino Acids as mentioned earlier are a class of 20 molecules that polymerize to form proteins. Proteins take on a large variety of roles, from catalyzing important reactions as enzymes, to providing structural support, such as collagen, to serving as hormone or neurotransmitter signals.
 |
 |
|
| L-alanine, an amino acid | A protein, a blue GPF varient, shown in stick form. | Ribbon view of the same protein. |
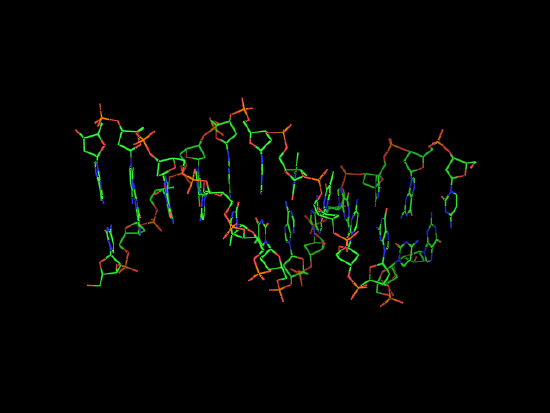
Nucleotides are a slightly more complex class of biological molecule, consisting of a phosphate group, a ribose or deoxyribiose sugar, and a nitrogenous base. Nucleotides, such as ATP serve as carriers of energy in a cell and provide energy to run processes that would otherwise be non-spontaneous. Nucleotides are able to polymerize into nucleic acids by forming a phosphodiester bond between the phosphate group of one nucleotide and a hydroxyl group on the ribose or deoxyribose of another nucleotide. The polymeric nucleic acids are able to store information for building proteins.
 |
|
| dATP, a nucleotide | B-DNA the polymer and a nucleic acid. |
Fatty Acids can already be considered somewhat polymeric, as they consist of a end carboxylic acid followed by a hydrocarbon chain of varying lengths. Three fatty acids can be attached to glycerol to form triglycerides. Other chemical groups can be added to the three hydroxyl groups of glycerol, thus yielding many chemical variations, which along with other non-polar chemicals are all included in the umbrella category of fats and lipids. Functions of these polymers again range from energy storage, to forming membranes necessary to structure, to signaling.
| palmitic acid, a fatty acid. | A triacylglycerol. |
Salts can be thought of as a polymers when considering the ionic crystal lattice. This can be applied to extracellular structures such as shells, teeth and bones. In cells, salts serve roles as ions in solution. Effects in this "monomeric" form include Ca2+ signaling, setting up electrochemical gradients to do work, or influencing osmotic properties.
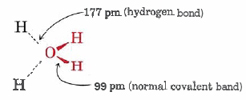
Water polymerizes in ice formation. Much of this structure still remains in liquid form, and hydrogen bonding networks still remain. So not only does water provide the solvent medium for all chemical reactions in cells, its structure dictates the way proteins fold, and how membranes form. It also maintains rigidity of cell walls, and serves as a thermoregulator. By virtue of its large polarity water is a good solvent for ionic substances and therefore provides a means of transporting inorganic nutrients such as NH4+, NO3–, CO32–, PO43–, and monatomic ions throughout higher organisms. Its ability to dissolve a wide variety of substances also makes it useful for disposing of wastes. Many of the human body’s defense mechanisms against external toxic substances involve conversion into water-soluble forms and elimination via urine.
 |
|
| A water molecule. | Ability to hydrogen bond allows for proton transfer, and confers structural elements to liquid water. |
Vitamins and trace elements, while not as prevalent as the chemical compounds discussed above, trace elements, such as zinc, copper, and iron are highly important to biological function, often serving key structural and chemical purposes in protein function. Vitamins are organic compounds that often augment proteins to help with biological functions.
| Vitamin B1, also known as thiamine. This organic molecule helps with the enzymatic activity of the protein pyruvate dehydrogenase complex. |
It is clear from this overview that both monomeric and polymeric forms of the chemical components of chemicals in living systems serve important functions. Also, those functions change depending on which building blocks are used for polymerization. However, the properties of these polymers can be understood in terms of the monomers and how they combine.


