19.15: Nuclear Power Plants
- Page ID
- 49619
Even before the atomic bomb had been produced, scientists and engineers had begun to think about the possibility of using the energy released by the fission process for the production of electrical energy. Shortly after World War II confident predictions were made that human beings would soon depend almost entirely on atomic energy for electricity. Alas, we are now years into the future from then and no such miracle has occurred. In the United States only 8.46 percent of the electrical energy in 2008 was produced by this method[1]. The proportion is a little higher in some other countries, notably Great Britain, but nowhere is nuclear power even on the verge of replacing the fossil fuels. The unfortunate truth is that producing power from atomic fission has turned out to be much more expensive than was previously expected. Even in these days of high prices for the fossil fuels it is still only barely competitive.
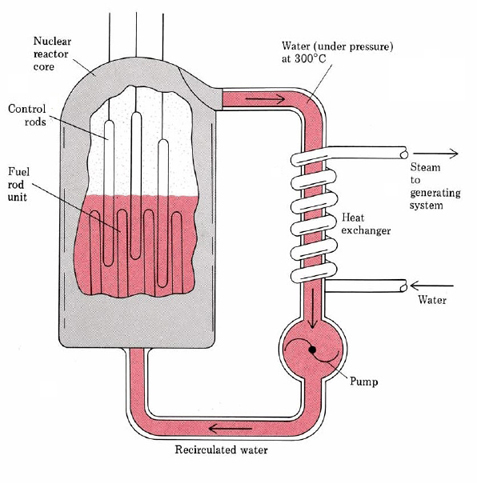
A schematic diagram of a typical nuclear reactor is given in Figure \(\PageIndex{1}\). The uranium is present in the form of pellets of the oxide U3O8 enclosed in long steel tubes about 2 cm in diameter. The uranium is23892U mainly, slightly enriched with the fissionable 23592U. The rate of fission can be regulated by inserting or withdrawing control rods made of cadmium, which is a very efficient neutron absorber. In addition a moderator such as graphite or water must be present to slow down the neutrons, since slow neutrons are more efficient at causing fission than fast ones.
The energy released by the fission of the uranium is carried off by a coolant, usually superheated steam at about 320°C. This steam cannot be used directly since it becomes slightly radioactive. Instead it is passed through a heat exchanger so as to produce further steam which can then be used to power a conventional steam turbine. The whole system is enclosed in a strong containment vessel which is not shown in the figure. This vessel prevents the spread of radioactivity in case of a serious accident.
Nuclear power plants have two advantages over conventional power plants using fossil fuels. First, for a given energy output they consume much less fuel. Second, they produce far smaller quantities of toxic effluents. Fossil-fueled plants produce sulfur dioxide, oxides of nitrogen, and smoke particles, all of which are injurious to health.
Despite the much lower cost for fuel, nuclear power plants are very expensive to build. This is largely because of their chief disadvantage, the extremely dangerous nature of the radioactive products of nuclear fission. Fission products consist of a great many neutron-rich, unstable nuclei, ranging in atomic number from 25 to 60. Particularly dangerous are the long-lived isotopes 9038Sr, 13755Cs, and the shorter-lived 13153I, all of which can be incorporated into the human body. Extreme precautions must be taken against accidental release of even traces of these materials into the environment. The worst case scenario is a reactor meltdown, of which the best known is the Chernobyl disaster, occurring in 1986 in Ukraine, then still a part of the Soviet Union. Recent study on the event estimates eventual deaths caused by the accident in the higher exposed populations as 4000 [2]. The overall cost of the disaster is of course difficult to quantify in terms of health, psychological, economic and environmental effects.
It should be realized that there are significant differences between a Hiroshima-type bomb and this sort of meltdown, primarily a difference in destructive explosive power. These differences arises because fuel used in nuclear reactors is not rich enough in23592U for the chain reaction to produce an atomic bomb-like explosion. It should also be noted that the safety deficiencies which caused the Chernobyl did not reflect the safety precautions taken in in nuclear plants today, or in other plants at the time. Regardless, the Chernobyl event, along with the earlier Three Mile Island incident in 1979, helped to start a decline in the use and building of nuclear power plants worldwide, and many people remain wary of nuclear power. Interest in nuclear power has renewed with discussions of national and world energy strategies, however.
Even if fission products are handled successfully during normal operation of a nuclear plant, there still remains the difficulty of their eventual disposal. Although many of the unstable nuclei produced by fission are short-lived, some, like9038Sr (25 years) and 13755Cs (30 years), have quite long half-lives. Accordingly these wastes must be stored for many hundreds of years before enough nuclei decompose to reduce their radioactivity to a safe level. At the present time, spent nuclear fuel is stored in spent fuel water pools at reaction sites, which shields the surrounding environment. Spent fuel may also be placed in dry cask storage after fuel has been stored in a spent fuel pool for at least a year. The dry casks contain inert gas, are made of steel, and may be surrounded by steel and concrete to prevent leakage of radiation [3].
Both spent fuel pools and dry cask storage are only short term solutions. One proposed long term solution is to store spent nuclear waste safely in a geological repository deep underground. In 1982, the Nuclear Waste Policy Act[4] tasked the United States Department of Energy with finding and constructing a national repository for long term storage of nuclear waste. In 1987, the Department of Energy was directed to focus solely on Yucca Mountain, Nevada, as the site to develop a national repository. Originally set to start accepting waste in 1998, the opening of the repository has been greatly delayed, often due to a large amount of unresolved debate on the topic.. Nevertheless, in 2002, the Department of Energy determined the site acceptable, and began application to use the site[5].
The Yucca Mountain Repository is currently out of favor. With shrinking budgets along with safety concerns about the site, the Department of Energy motioned to withdraw the Yucca Mountain site as a long term repository for nuclear waste in 2010[6]. The debacle over Yucca Mountain highlights both the scientific and political difficulties in finding a long term solution to spent nuclear fuels, as well as the difficulties involved in using nuclear power in general.
References
- "Annual Energy 2008: Energy Flow" Energy Information Administration. 5 August 2009. www.eia.doe.gov/emeu/aer/pdf/pages/sec1_3.pdf
- "Chernobyl’s Legacy: Health, Environmental and Socio-Economic Impacts and Recommendations to the Governments of Belarus, the Russian Federation and Ukraine" The Chernobyl Forum. 2003-2005. http://www.iaea.org/Publications/Boo.../chernobyl.pdf
- "Storage of Spent Nuclear Fuel" United States Nuclear Regulatory Commission. 2010. http://www.nrc.gov/waste/spent-fuel-storage.html
- "Nuclear Waste Policy Act" United States Department of Energy. Amended 2004. http://web.archive.org/web/20080514020437/http://www.ocrwm.doe.gov/documents/nwpa/css/nwpa.htm
- "Fact Sheet on Licensing Yucca Mountain" United States Nuclear Regulatory Commission. 2009. www.nrc.gov/reading-rm/doc-co...nse-review.pdf
- US Department of Energy's Motion to Withdraw. US Department of Energy. 2010. www.energy.gov/news/documents...o_Withdraw.pdf.


