18.2: The Rate of Reaction
- Page ID
- 49581
A chemist who investigates the rate of a reaction often wants to see how the rate differs at two different temperatures. In the video below, this situation is explicitly investigated with the glucose oxidase enzyme reaction of glucose to gluconolactone, which is visualized by a dye which turns to a blue-green color as the reaction proceeds. One reaction is run at 22°C and the other at 4°C. The reaction clearly proceeds more quickly at 22°C, which gains a darker blue-green color more quickly than the reaction run at 4°C.
Maybe instead of being run at two different temperatures, a reaction has an added catalyst, which is a compound which does not change in the reaction, but causes the reaction to go through a different mechanism. The following video shows two cases of the reaction:
\[\text{ 2MnO}_{\text{4}}^{-}(aq) + \text{ 5H}_{\text{2}}\text{C}_2\text{O}_{\text{4}}(aq) + \text{ 6H}_{\text{3}}\text{O}^{+}(aq)\rightarrow \text{2Mn}^{2+}(aq) + \text{ 10CO}_{\text{2}}(aq) + \text{ 14H}_{\text{2}}\text{O} \nonumber \]
Manganese(II) sulfate is added as a catalyst to the solution on the right, which increases the rate of reaction. This solution changes from a dark pink solution to a clear solution far more quickly than the solution on the left.
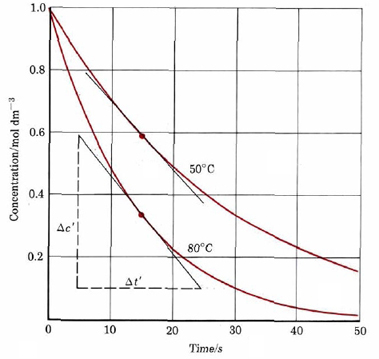
In order think meaningfully about these different rates of reaction, the rate needs to be quantified. This can be done for reactions in solution by looking at the dependence of concentration of a reactant or product when considering time. Figure 1 displays a decomposition reaction of dye into colorless chemicals at two different temperatures, giving dye concentration at different times.
As an example of the use of this definition of reaction rate, consider the first 10 s during decomposition of the dye at 50°C. According to Figure 1 the concentration of dye drops to 0.70 mol dm–3 from an initial value of 1.0 mol dm–3. If we represent the change in the concentration of the reactant dye by ΔcR, then
ΔcR = c1 – c2 = (0.70 – 1.00) mol dm–3 = – 0.30 mol dm–3
In other words the concentration of dye has decreased by 0.30 mol dm–3. We can calculate an average rate of reaction by dividing this concentration decrease by the time interval (Δt = t2 – t1 = 10 s – 0 s = 10 s) during which it occurred:
Average rate = 0.30 mol dm–3 10–1 s–1 = 0.03 mol dm–3 s–1
Clearly, no matter what reactant we observe, its concentration will decrease with time and ΔcR will be negative. On the other hand, the concentration of a reaction product will always increase, and so ΔcP will always be positive. Since we want the average reaction rate always to be positive, we define it as
\[\text{Rate = }\dfrac{-\Delta c_{R}}{\Delta t} = \dfrac{\Delta c_{P}}{\Delta t} \nonumber \]
Calculate the average rate of decomposition of the dye in Figure 1 at 80°C during the time interval(a) 0 to 10 s; (b) 10 to 20 s; (c) 20 to 30 s; (d) 0 to 30 s.
Solution
a) In the interval 0 to 10 s,
Δt = 10 s – 0 s = 10 s
\(\text{Rate = }\dfrac{-\Delta c_{R}}{\Delta t}\) = – \(\dfrac{-\text{(}-\text{0}\text{.51 mol dm}^{-\text{3}}\text{)}}{\text{10 s}}\) = 0.051 mol dm–3 s–1
b) \(\text{Rate = }\dfrac{-\Delta c_{R}}{\Delta t}\) = \(\dfrac{-\text{(0}\text{.24 }-\text{0}\text{.49) mol dm}^{-\text{3}}}{\text{(20}-\text{10) s}}\) = 0.025 mol dm–3 s–1c) \(\text{Rate = }\dfrac{-\Delta c_{R}}{\Delta t}\) = \(\dfrac{-\text{(0}\text{.11 }-\text{0}\text{.24) mol dm}^{-\text{3}}}{\text{(30}-2\text{0) s}}\) = 0.013 mol dm–3 s–1
d) \(\text{Rate = }\dfrac{-\Delta c_{R}}{\Delta t}\) = \(\dfrac{-\text{(0}\text{.11 }-1.\text{00) mol dm}^{-\text{3}}}{\text{(30}-\text{0) s}}\) = 0.030 mol dm–3 s–1
This example illustrates two important points about reaction rates. The first is that the rate of a reaction usually decreases with time, reaching a value of zero when the reaction is complete. This is usually because the rate depends in some way on the concentrations of one or more reactants, and as those concentrations decrease, the rate also decreases. The second point is a corollary to the first. Because the reaction rate changes with time, the rate we measure depends on the time interval used. For example, the average rate over the period to 30 s was 0.030 mol dm–3 s–1, but the average rate over the middle 10 s of that period was 0.025 mol dm–3 s–1. The different average for the 30-s interval reflects the fact that the rate dropped from 0.051 to 0.013 mol dm–3 s–1 during that period.
To measure a reaction rate as accurately as possible at a particular time, we need to make the time interval Δt as small as we can so that there is the least possible change in rate over the interval. If you are familiar with the branch of mathematics known as the differential calculus, you will recognize that as Δt becomes smaller, Δc/Δt approaches a limit known as the derivative. Thus the rate of reaction can be defined exactly as
\[\text{Rate = }\dfrac{-dc_{R}}{dt} = \dfrac{dc_{P}}{dt} \nonumber \]
The derivative also gives the slope of the tangent to a graph of versus t. Such a graph is shown in Figure \(\PageIndex{1}\), with a tangent line drawn at t = 15 s. The slope of this line is – 0.0245 dm–3 s–1, giving
-
- \(\text{Rate = }\dfrac{-dc_{R}}{dt}\) = – slope of tangent = 0.0245 mol dm–3 s–1
This exact value is quite close to the average rate of 0.025mol mol dm–3 s–1 calculated for the interval 10 to 20 s, but it is farther from the 0.030 mol dm–3 s–1 calculated over the 0 to 30-s interval. As expected, the larger the time interval, the less accurate the calculated rate.
Reaction Rate and Equation Coefficients
The stoichiometry of the reaction in which the dye decomposes has a simple equation
Dye → colorless products
in which the coefficient of the reactant dye is one. When some coefficients are larger than one, there is one additional aspect of defining the rate of reaction. Consider the reaction
\[\text{ 2N}_{\text{2}}\text{O}_5(g)\rightarrow \text{ 4NO}_{\text{2}}(g) + \text{O}_{\text{2}}(g) \nonumber \]
According to stoichiometry, two molecules of N2O5 must disappear for every one molecule of O2 that is formed. And for every mole of O2 formed, there must be four moles of NO2 formed. Thus NO2 is formed four times as fast as O2, and N2O5 disappears twice as fast as O2 appears. If we define the rate in terms of the change in concentration of NO2 per unit time, then the rate will be four times faster than if we define the rate in terms of the change in concentration of O2. Similarly, the rate of disappearance of N2O5 will be twice as great as the rate of appearance of O2.
Having the rate of reaction differ depending on which substance we consider could cause a lot of confusion. Therefore, by convention, the rate is defined in a way that always gives the same value. This is done by multiplying the appropriate derivative by the reciprocal of the coefficient in the balanced chemical equation. For the reaction above, the rate is defined as
\[Rate = -\dfrac{1}{2}\dfrac{d[N_2 O_5]}{dt}=\dfrac{1}{4}\dfrac{d[NO_2 ]}{dt}=\dfrac{1}{1}\dfrac{d[O_2]}{dt} \nonumber \]
Although N2O5 is disappearing twice as fast as O2 is forming (that is, d[N2O5]/dt is twice d[O2]/dt), multiplying d[N2O5]/dt by the factor 1/2 and changing its sign insures that the rate has the same value. Similarly, even though d[NO2]/dt is four times as great as d[O2]/dt, the factor of 1/4 makes the rate the same.
Contributors
Ed Vitz (Kutztown University), John W. Moore (UW-Madison), Justin Shorb (Hope College), Xavier Prat-Resina (University of Minnesota Rochester), Tim Wendorff, and Adam Hahn.


