17.3: Electrolysis of Brine
- Page ID
- 49551
Three important chemicals, NaOH, Cl2, H2, can be obtained by electrolyzing an aqueous NaCl solution (brine). This forms the basis of the chlor-alkali industry. The diaphragm cell (also called a Hooker cell) in which the electrolysis is carried out is shown schematically in Figure \(\PageIndex{1}\). At the cathode, water is reduced:
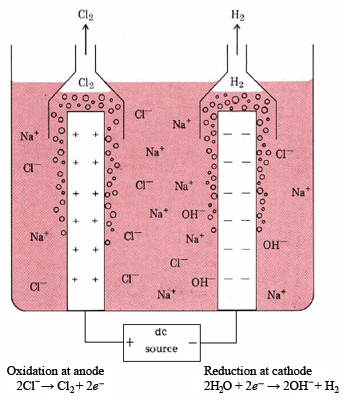
\[\ce{2H2O + 2} e^{-} \ce{ -> H2 + 2OH^-} \nonumber \]
Chlorine is produced at the anode:
\[\text{2Cl}^{-} \rightarrow \text{Cl}_2 + 2e^{-} \nonumber \]
Thus the overall reaction is
\[\text{2H}_2\text{O}(l) + \text{2Cl}^{-}(aq) \rightarrow \text{H}_2(g) + \text{Cl}_2(g) + \text{2OH}^{-}(aq)\label{3} \]
Since the H2(g) and Cl2(g) might recombine explosively should they come in contact, the cathode must be entirely surrounded by a porous diaphragm of asbestos. Hence the name of this type of cell.
Both the H2(g) and Cl2(g) produced in Eq. \(\ref{3}\) are dried, purified, and compressed into cylinders. Fresh brine is continually pumped into the cell, and the solution which is forced out contains about 10% NaOH together with a good deal of NaCl. [Remember that the spectator ions, Na+(aq), are not included in a net ionic equation such as Eq. \(\ref{3}\). H2O is allowed to evaporate from this solution until the concentration of the solution reaches 50% NaOH, by which time most of the NaCl has crystallized out and can be recycled to the electrolysis. The NaOH is sold as a 50% solution or further dried to give crystals whose approximate formula is NaOH•H2O.
The considerable effort required to concentrate the NaOH solution obtained from diaphragm cells can be avoided by using mercury cells. The cathode in such a cell is mercury, and the cathode reaction is
\[\text{Na}^{+}(aq) + e^{-} + \text{xHg}(l) \rightarrow \text{NaHg}_x(l) \nonumber \]
The sodium metal produced in this reaction dissolves in the liquid mercury, producing an amalgam. The liquid amalgam is then transferred to an- other part of the cell and reacted with water:
\[\text{NaHg}_x(l) + \text{2H}_2\text{O}(l) \rightarrow \text{2Na}^{+}(aq) + \text{2OH}^{-}(aq) + \text{H}_2(g) + \text{xHg}(l) \nonumber \]
The 50% sodium hydroxide solution produced by this reaction contains no sodium chloride and can be sold directly, without being concentrated further. Up until 1970, however, chlor-alkali plants using mercury cells did not have adequate controls to prevent losses of mercury to the environment. About 100 to 200 g mercury was lost for each 1000 kg chlorine produced-apparently a small quantity until one realizes that 2 500 000 kg chlorine was produced by mercury cells every day during 1960 in the United States. Thus every 2 to 4 days 1000 kg mercury entered the environment, and by 1970 sizable quantities were being found in fish. Since 1970 adequate controls have been installed on mercury cells and most new alkali plants use diaphragm cells, but the very large quantities of mercury introduced into rivers and lakes prior to 1970 are expected to remain for a century or more.


