11.18: Common Oxidizing Agents
- Page ID
- 49545
Oxidizing agents must be able to accept electrons readily. Elements with high electronegativity readily accept electrons, as can molecules or ions which contain relatively electronegative elements and even some metals which have high oxidation numbers. Bear these general rules in mind as we examine examples of common oxidizing agents in the following paragraphs.
Oxidizing Agents
Halogens (group VllA elements)
All four elemental halogens, F2, Cl2, Br2, and I2, are able to accept electrons according to the half-equation
\[\ce{X2 + 2e^{–} -> 2X^{–}} \nonumber \]
with \(X = F, Cl, Br, I\)
As we might expect from the periodic variation of electronegativity, the oxidizing power of the halogens decreases in the order F2 > Cl2 > Br2 > I2. Fluorine is such a strong oxidizing agent that it can react with water (water is very difficult to oxidize):
\[\ce{2F2 + 6H2O -> 4H3O^+ + 4F^{–} + O2} \nonumber \]
Chlorine also reacts with water, but only in the presence of sunlight. Bromine is weaker, and iodine has only mild oxidizing power.
Oxygen
Oxygen gas, which constitutes about 20 percent of the earth’s atmosphere, is another electronegative element which is a good oxidizing agent. It is very slightly weaker than chlorine, but considerably stronger than bromine. Because the atmosphere contains such a strong oxidant, few substances occur in reduced form at the earth’s surface. An oxidized form of silicon, SiO2, is one of the most plentiful constituents of the crust of the earth. Most metals, too, occur as oxides and must be reduced before they can be obtained in elemental form. When iron rusts, it forms the red-brown oxide Fe2O3•xH2O, seen below, which always contains an indeterminate amount of water.

Iron Oxide (Fe2O3•xH2O)
Oxyanions and oxyacids
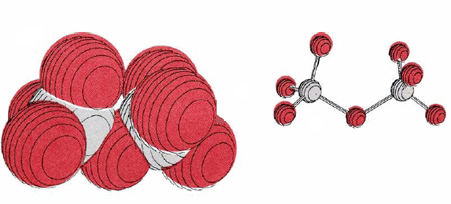
In aqueous solution NO3–, IO3–, MnO4–, Cr2O72–, and a number of other oxyanions serve as convenient, strong oxidizing agents. The structure of the last oxyanion mentioned above is shown in Figure 1. The most strongly oxidizing oxyanions often contain an element in its highest possible oxidation state, that is, with an oxidation number equal to the periodic group number. For example, NO3– contains nitrogen in a +5 oxidation state, Cr2O72– (seen below) contains chromium +6, and has manganese +7. The oxidizing power of the dichromate ion is employed in laboratory cleaning solution, a solution of Na2Cr2O7 in concentrated H2SO4. This readily oxidizes the organic compounds in grease to carbon dioxide. It is also highly corrosive, eats holes in clothing, and must be handled with care. Dark purple permanganate ion is another very common oxidizing agent (seen below). In basic solution it is reduced to solid dark brown MnO2. In acidic solution, however, it forms almost colorless Mn2+(aq).