8.22: Silicon Dioxide
- Page ID
- 49475
Silicon dioxide, or silica, (SiO2) is another important example of a macromolecular solid. Silica can exist in six different crystalline forms. The best known of these is quartz, whose crystal structure shown previously is shown again below.
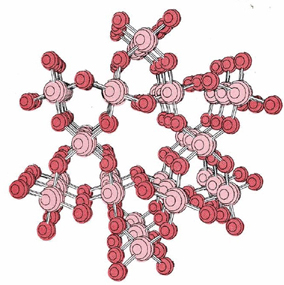
Sand consists mainly of small fragments of quartz crystals. Quartz has a very high melting point, though not so high as diamond.
If you refer back to the examples on silicon, you can remind yourself of the reason that SiO2 is macromolecular. Silicon is reluctant to form multiple bonds, and so discrete 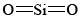 molecules, analogous to
molecules, analogous to 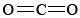 , do not occur. In order to satisfy silicon’s valence of 4 and oxygen’s valence of 2, each silicon must be surrounded by four oxygens and each oxygen by two silicons. This can be represented schematically by the Lewis diagram
, do not occur. In order to satisfy silicon’s valence of 4 and oxygen’s valence of 2, each silicon must be surrounded by four oxygens and each oxygen by two silicons. This can be represented schematically by the Lewis diagram


