8.7: Cycloalkanes
- Page ID
- 49432
Though petroleum consists mainly of normal and branched alkanes, other classes of compounds also occur. The next best represented are the cycloalkanes, which are characterized by at least one ring of carbon atoms. If a hydrocarbon chain is to be made into a ring, a new C—C bond must be formed between carbon atoms at the end of the chain. This requires that two hydrogen atoms be removed to make room for the new bond. Consequently such a ring involves one more C—C bond and two less C—H bonds than the corresponding normal alkane, and the general formula for a cycloalkane is CnH2n. The most common cycloalkane in petroleum is methylcyclohexane, which has the projection formula
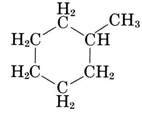
A ball-and-stick model of one of the conformations of methylcyclohexane is shown in the figure below. Note that the ring of six carbon atoms is puckered to permit tetrahedral arrangement of bonds around the carbon atoms. Also shown in the figure is cyclopentane, C5H10, which contains a five-membered ring. Virtually all the cycloalkanes in petroleum contain five- or six-membered rings similar to those illustrated.
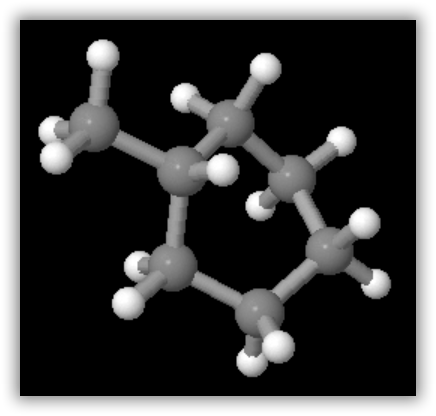 Methylcyclohexane, C7H14, the most common cycloalkane found in crude oil. Note that the six-membered ring is puckered to accommodate the tetrahedral bond angles of carbon.
Methylcyclohexane, C7H14, the most common cycloalkane found in crude oil. Note that the six-membered ring is puckered to accommodate the tetrahedral bond angles of carbon.
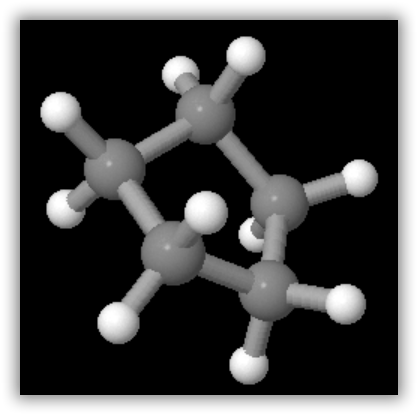
Cyclopentane, C5H10, in which the ring of carbon atoms is more nearly planar but is still slightly puckered.


