8.16.2: Cultural Connections- The Cinnamon Trade
- Page ID
- 50828
Cinnamon has been known for thousands of years. One of the first times it is mentioned is in the Old Testament when Moses uses cinnamon in holy oil. Using cinnamon in holy oil shows how prized this spice was in ancient times. In fact, in Greece cinnamon was used as a sacrifice to the Gods like Apollo. Although cinnamon was used in the Mediterranean, it originated in Sri Lanka. The cinnamon traveled along the spice route, and the actual origins of cinnamon were held quiet. By not allowing other traders to know the source of the spice the growers were able to keep a monopoly and control the price (at a high one of course). In the sixteenth century Portugal finally made it to Sri Lanka. In 1518 a trading fort was set up there. The Dutch fought the Portuguese and eventually won control of the cinnamon trade in 1658. The British eventually took control of the island in 1796 but by then cinnamon wasn't as highly prized.
Through the spice trade cinnamon made its way out of Sri Lanka and into the cuisines and cultures of the rest of the world. In Mexico especially cinnamon is a huge part of their cuisine. In fact, Mexico is the main importer of raw cinnamon. The majority of this cinnamon is used in the processing of chocolate. Also, cinnamon in Mexico is used in desserts, hot chocolate, and many other things. The spice is used in the same kind of dishes in the United States, as well as things such as cereal. In the Middle East cinnamon is used to flavor lamb as well as soups and even as a flavor in pickling. Cinnamon's unique taste and ability to be used as a spice is the reason why it has been incorporated into cultures all around the world. The component in cinnamon that gives it it's properties is the presence of cinnamaldehyde.
The essential oil that makes up cinnamon is over 90% cinnamaldehyde. Cinnamaldehyde is an organic compound that can also be classified as an aldehyde. Cinnamaldehyde is unique in that it also contains a benzene ring and a double bond, as is seen in the structure in Figure 1. Cinnamaldehyde is also used in many other foods as a flavoring. While cimmaldehyde can be synthesized, the main source of it is from cinnamon bark.
More on Aldehydes and Ketones
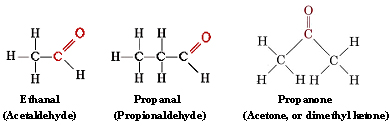
The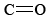 functional group found in formaldehyde is called a carbonyl group. Two classes of compounds may be distinguished on the basis of the location of the carbonyl group. In aldehydes it is at the end of a carbon chain and has at least one hydrogen attached. In ketones the carbonyl group is attached to two carbon atoms. Some examples are
functional group found in formaldehyde is called a carbonyl group. Two classes of compounds may be distinguished on the basis of the location of the carbonyl group. In aldehydes it is at the end of a carbon chain and has at least one hydrogen attached. In ketones the carbonyl group is attached to two carbon atoms. Some examples are
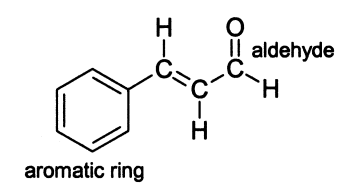 cinnamaldehyde
cinnamaldehyde
Chemical tests may be performed to determine the presence of a carbonyl group. In the video below, a solution of 2,4-dinitrophenylhydrazine is added to test tubes containing 2-propanol, an alcohol; 2-propanone (acetone), a ketone; and propionic acid a carboxylic acid. 2,4-dinitrophenylhydrazine only reacts with the carbonyl group of 2-propanone, forming an orange precipitate.
The reaction that occurs is:
Another test can distinguish between aldehydes and ketones. Here is a video of the Silver Mirror Tollens Test for Aldehydes:
Tollens reagent is an aqueous solution of silver nitrate, sodium hydroxide, and a little ammonia. (In the video the ammonia comes from reaction of ammonium ions in ammonium nitrate and hydroxide ions from sodium hydroxide to form water and NH3.) If an aldehyde, in this case, glucose, is added to the solution, the Ag+ is reduced by the aldehyde, and the aldehyde is oxidized into a carboxylic acid. This produces silver metal, which coats the flask and creates the mirror. A similar reaction does not occur for ketones, so only aldehydes produce the silver mirror. The equation for the reaction in the video is:
The endings al and one signify aldehyde and ketone, respectively. The general formula for an aldehyde is ,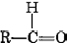 while for a ketone it is . Note that every ketone is isomeric with at least one aldehyde. Acetone, for example, has the same molecular formula (C3H6O) as
while for a ketone it is . Note that every ketone is isomeric with at least one aldehyde. Acetone, for example, has the same molecular formula (C3H6O) as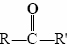 propanal.
propanal.
Aldehyde and ketone molecules cannot hydrogen bond among themselves for the same reason that ethers cannot—they do not contain hydrogens attached to highly electronegative atoms. The carbonyl group is rather polar, however, since the difference between the electronegativities of carbon (2.5) and oxygen (3.5) is rather large, and there are usually no other dipoles in an aldehyde or ketone molecule to cancel the effect of C==O.
Therefore the boiling points of aldehydes and ketones are intermediate between those of alkanes or ethers on the one hand and alcohols on the other. Acetaldehyde, CH3CH2CHO, boils at 20.8°C midway between propane (–42°C) and ethanol (78.5°C). The boiling points of propanal and acetone are compared with other organic compounds in the table of the boiling points of comparable organic compounds which shows the same trend.
Of all the aldehydes and ketones, formaldehyde and acetone are of greatest commercial importance. Uses of formaldehyde have already been mentioned. Like other ketones, acetone is mainly useful as a solvent, and you may have used it for this purpose in the laboratory. Acetone and other ketones are somewhat toxic and should not be handled carelessly.
From ChemPRIME: 8.15: Aldehydes and Ketones


