16.4: Toxic Substances and Toxicology
- Page ID
- 285401
One of the greater concerns that the general public has with chemistry is the potential toxic effects of various substances including those that could be used for terrorist attacks. Poisons, or toxicants, are substances that can adversely affect biological tissue leading to harmful responses including, in the severest cases, even death. The study of such substances and their effects is the science of toxicology. The science that relates the chemical properties of toxic substances to their toxic effects is toxicological chemistry. Because poisons are among the leading terrorist threats, it is appropriate to consider toxic substances and toxicological chemistry here.
Any kind of tissue and all organs can be the subject of attack by toxic substances. The major human organ systems that are potentially adversely affected by toxic substances are given in Table \(\PageIndex{1}\).
| Table \(\PageIndex{1}\) | Typical Toxic Responses |
|---|---|
| Respiratory system | Emphysema from cigarette smoke, lung cancer from asbestos |
| Skin responses | Allergic contact dermatitis, such as from exposure to dichromate; chloracne from exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (“dioxin”); skin cancer from exposure to coal tar constituent |
| Hepatotoxicity (toxic effects) | Steatosis (fatty liver), such as from exposure to carbon tetrachloride cirrhosis; (deposition and build up of fibrous collagen tissue) from excessive ingestion of ethanol; haemangiosarcoma, a type of liver cancer observed in workers heavily exposed to vinyl chloride in PVC plastic manufacture |
| Reproductive system | Interference with sperm development by some industrial chemicals, interference with cells involved with egg formation by chemicals such as cyclophosphamide |
|
Blood |
Carboxyhemoglobin formation from binding of carbon monoxide to blood hemoglobin, methemoglobinemia consisting of conversion of iron(II) to iron(III) in hemoglobin from exposure to substances such as aniline or nitrobenzene, aplastic anemia from exposure to benzene |
| Immune system effects | Immunosuppression from exposure to radiation, hypersensitivity from exposure to beryllium |
| Endocrine system effects | Disruption of endocrine function by endocrine disruptors such as bisphenol-A |
| Nervous system | Encephelopathy (brain disorder), such as from exposure to lead; peripheral neuropathy from exposure to organic solvents; inhibition of acetylcholinesterase enzyme in nerve function by exposure to organophosphate military poisons |
| Kidney and urinary tract system | Nephrotoxicity to the kidney by heavy metal cadmium |
Toxicities
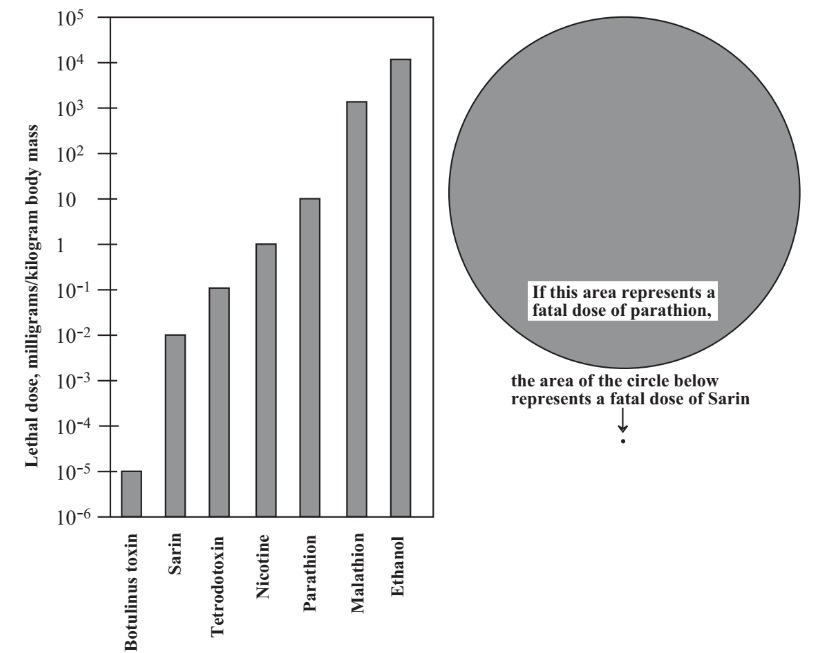
The toxicities of substances vary over a wide range, and those that are toxic at lowest doses are of most concern for deliberate poisoning. This is illustrated in Figure 16.1 which gives the toxicities of several substances. It is important to note that the dosage scale in this figure is logarithmic; that is for each division decrease on the scale, a substance is ten times as toxic. The two circles in Table \(\PageIndex{1}\) illustrate the enormous differences between toxicities of different substances. If the area of the large circle represents the size of a fatal dose of parathion, a once widely used insecticide that has killed a number of people and has now been banned because of its toxicity, a fatal dose of military poison nerve gas Sarin is represented by the minuscule dot below the circle! Toxicities are normally expressed as LD50 values, the dose in units of mass of poison per unit mass of test subject. Rats are usually used for tests, and toxicities to humans are inferred from these test values.
Metabolism of Toxic Substances
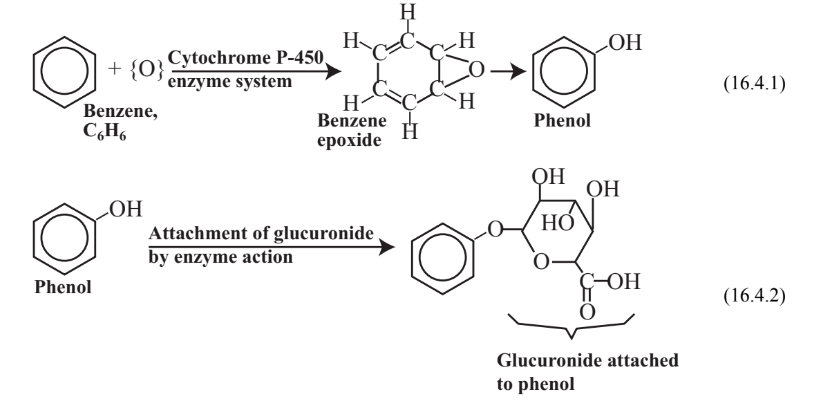
Toxic substances that enter the body and that are foreign to it, commonly called xenobiotic substances, are subject to metabolic processes that may activate them or make them less toxic(detoxification). The metabolism of toxic substances may be divided into two phases. Phase I reactions normally consist of attachment of a functional group, usually accompanied by oxidation. For example, benzene, C6H6, (see Chapter 6, Section 6.2) is oxidized in the body by the action of the cytochrome P-450 enzyme system as shown in Reaction 16.4.1. The Phase I oxidation product is phenol, a toxic substance. A reactive intermediate in the process is benzene epoxide, which interacts with biomolecules to cause toxic effects. The phenol Phase I oxidation product of benzene may undergo a second reaction, a Phase II reaction in which it is bound with a conjugating agent that is endogenous to (produced naturally by) the body, such as glucuronide as illustrated in Reaction 16.4.2
Although Phase I and Phase II reactions generally act to make xenobiotic substances more water soluble, more readily eliminated from the body, and less toxic, in some cases, the opposite occurs and metabolic processes make substances more toxic. Most known human carcinogens (cancer-causing agents) are actually produced by biochemical processes in the body from noncarcinogenic precursor substances.
The Action of Toxic Substances
Toxic substances, which, as noted above, are often produced by metabolic processes from nontoxic precursors, produce a toxic response by acting upon a receptor in the body. Typically, a receptor is an enzyme that is essential for some function in the body. As a consequence of the binding of the receptor to the toxicant there is a biochemical effect. A common example of a biochemical effect occurs when a toxicant binds to an enzyme such that the bound enzyme may be inhibited from carrying out its normal function. As a result of a biochemical effect, there is a response, such as a behavioral or physiological response, which constitutes the actual observed toxic effect. Acetylcholinesterase enzyme inhibited by binding to nerve gas Sarin may fail to stop nerve impulses in breathing processes, leading to asphyxiation. The phenomena just described occur in the dynamic phase of toxicant action as summarized in Figure 16.3.


