10.5: Energy and Mass Transfer in the Atmosphere
- Page ID
- 285351
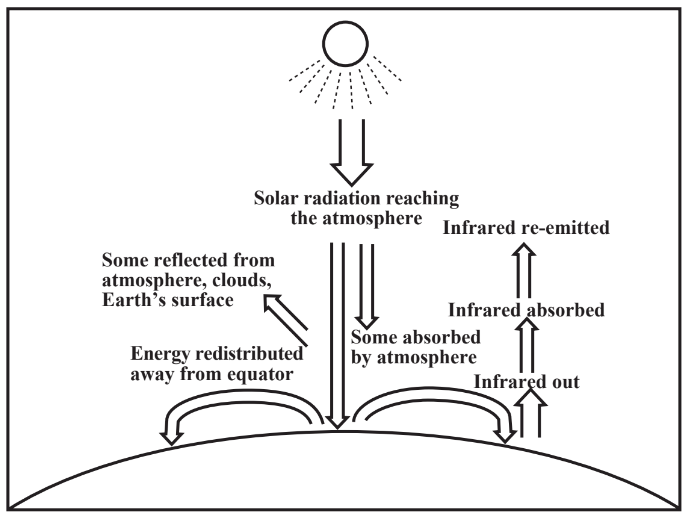
The flux of energy reaching Earth’s atmosphere from the sun as sunlight is 1,340 watts/m2.This means that a square meter of area perpendicular to incoming sun rays above Earth’satmosphere is receiving solar energy at a rate sufficient to power 13 100-watt light bulbs plus a 40-watt bulb, enough to power an electric iron or a hair dryer set on high! This is an enormous amount of energy. As shown in Figure 10.3, some of the incoming energy reaches Earth’s surface, some is absorbed in the atmosphere, warming it, and some is scattered back to space. The energy that comes in primarily as light at a maximum intensity of 500 nanometers in the visible region must go out, which it does as infrared radiation (with maximum intensity at about 10 micrometers(μm), primarily between 2μm and 40μm). Water molecules, carbon dioxide, methane, and other minor species in the atmosphere absorb some of the outbound infrared, which eventually is all radiated to space. This temporary absorption of infrared radiation warms the atmosphere — a greenhouse effect.
The fraction of electromagnetic radiation from the sun that is reflected by Earth’s surface varies with the nature of the surface. The percentage reflected is very important because it determines how effective incoming radiation is in warming the surface and it is expressed as albedo. Freshly plowed black topsoil has a very low albedo of only about 2.5%. In contrast, the albedo of a covering of fresh snow is about 90%. The anthrosphere affects albedo. One of the ways that this is done is in cultivating land, turning over relatively high albedo grass and covering it with exposed black soil that absorbs light energy very strongly. Another way is covering of large areas with asphalt paving, which reflects sunlight poorly.
The maintenance of Earth’s heat balance to keep temperatures within limits conducive to life is very complex and not well understood. Geological records show that in times past, Earth was sometimes relatively warm and that at other times there were ice ages in which much of Earth’ssurface was covered by ice a kilometer or two thick. The differences in average Earth temperature between these extremes and the relatively temperate climate conditions that we now enjoy were only a matter of a few degrees. It is also known that massive volcanic eruptions and almost certainly hits by large asteroids have caused cooling of the atmosphere that has lasted for a year or more. As addressed later in this chapter, there is now concern that anthropogenic gas emissions, particularly of carbon dioxide from fossil fuel combustion, may be having a warming effect upon the atmosphere.
Earth receives solar energy most directly at the equator, so equatorial regions are warmer than regions farther north and south. A significant fraction of this energy moves away from the equator. This is largely done by convection in which heat is carried by masses of air. Such heat can be in the form of sensible heat from the kinetic energy of rapidly moving air molecules (the faster their average velocities, the higher the temperature). Heat can also be carried as latent heat in the form of water vapor. The heat of vaporization of water is 2,259 joules per gram (J/g) meaning that 2,259 joules of heat energy are required to evaporate a gram of water without raising its temperature. This is a very high value, meaning that the evaporation of ocean water by solar energy falling on it in warmer regions absorbs an enormous amount of heat to form water vapor. This vapor may be carried elsewhere and condense to form rainfall. The heat energy released raises the temperature of the surrounding atmosphere.
Meteorology
The movement of air masses, cloud formation, and precipitation in the atmosphere are covered by the science of meteorology. Meteorologic phenomena have a strong effect upon atmospheric chemistry by processes such as the following:
• Movement of air pollutants from one place to another, such as the movement of air pollutant sulfur dioxide from the U.S. Ohio River Valley to New England and southern Canada, where it forms acid rain.
• Conditions under which stagnant pollutant air masses remain in place so that secondary pollutants, such as photochemical smog, can form.
• Precipitation, which can carry acidic compounds from the atmosphere to Earth’s surface in the form of acid rain
Atmospheric chemical processes can influence meterological phenomena. The most obvious example of this is the formation of rain droplets around pollutant particles in the atmosphere.
Weather refers to relatively short term variations in the state of the atmosphere as expressed by temperature, cloud cover, precipitation, relative humidity, atmospheric pressure and wind. Weather is driven by redistribution of energy in the atmosphere. A particularly important aspect of this redistribution is the energy released when precipitation forms. This energy can be enormous because of the high heat of vaporization of water. As an example, heat energy from sunlight and from hot masses of air is converted to latent heat by the evaporation of ocean water off the west coast of Africa. Prevailing winds drive masses of air laden with water vapor westward across the ocean. Rainfall forms, releasing the energy from the latent heat of water and warming the air mass. The hot mass of air that results rises, creating a region of low pressure into which air flows in a circular manner. This can result in the formation of a whirling mass of air in the form of a hurricane that may strike Puerto Rico, Cuba, Florida, or other areas thousands of miles from the area where the water was originally evaporated from the ocean.
A very obvious manifestation of weather consists of very small droplets of liquid water composing clouds. These very small droplets may coalesce under the appropriate conditions to form raindrops large enough to fall from the atmosphere. Clouds may absorb infrared radiation from Earth’s surface, warming the atmosphere, but they also reflect visible light, which has a cooling effect. Pollutant particles are instrumental in forming clouds. One of the more active kinds of cloud-forming pollutants are atmospheric strong acids, particularly H2SO4,
A lack of wind and air currents often occurs under conditions of temperature inversion in which warmer air masses overlay cooler ones (see Figure 10.1). As shown in this figure, topographical features, such as a mountain range that limits horizontal air movement, may make temperature inversion much more effective in trapping polluted masses of air. These conditions occur in the Los Angeles basin noted for photochemical smog formation.


