10.3: The Protective Atmosphere
- Page ID
- 285349
The atmosphere is the air around and above us. We know we must have air to breathe. A human deprived of air’s life-giving oxygen for just a brief time will lose consciousness, and within a few minutes will die. But air is far more than just a source of oxygen. That is because it protects Earth’s organisms in ways that are absolutely essential for their existence. One major protective function is to act as a blanket to keep us warm. It does that by reabsorbing the infrared radiation by which Earth radiates the energy that it receives from the sun. By delaying the exit of this energy into outer space, the average temperature of Earth’s surface remains at about 15 ̊C at sea level, though much colder at certain times and places and significantly warmer at others. Without this warming effect, plants could not grow and most other known organisms could not exist. The second protective function of the atmosphere is absorption of very short wavelength ultraviolet solar radiation. Were this radiation to reach our level, it would tear apart biomolecules, making it impossible for most life forms to exist.
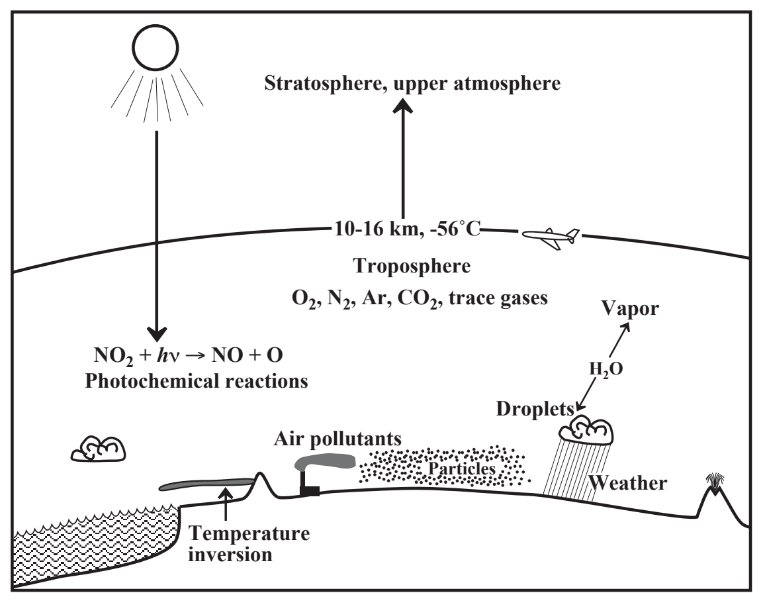
Although one might get the impression that the atmosphere is very thick, it is “tissue thin” compared to Earth’s diameter. Consider a corporate jet aircraft cruising at 35,000 feet (about 6.6miles or 10.7 kilometers). In the unlikely event of sudden, catastrophic loss of pressure in the pressurized cockpit, the pilot has only about 15 seconds to grab an oxygen mask before losing consciousness (the passengers in the cabin have an equally short time, but it is more important for the pilot to stay conscious and dive to a lower altitude). The reason for this is that virtually all the air in the atmosphere is below the altitude of around 11 km. By way of comparison, Earth’sdiameter is almost 13,000 km.
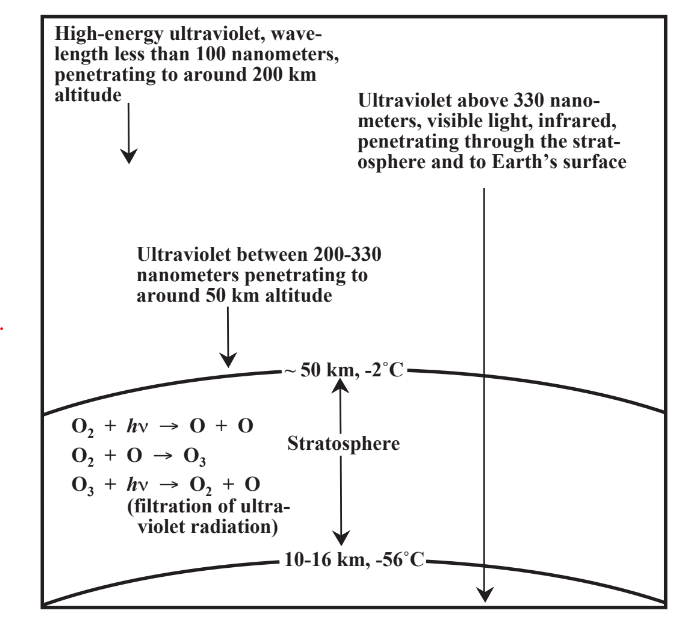
The altitude at which high-flying jet aircraft cruise marks the upper limit of the lowest of several layers of the atmosphere, the troposphere, which extends from sea level to about 11 km (Figure 10.1). As anyone who has driven to the top of Pike’s Peak or some other mountain knows, the troposphere gets cooler with increasing altitude, from an average temperature of 15 ̊C at sea level to an average at 11 km of -56 ̊C. Above the layer of the troposphere, however, atmospheric temperature increases to an average of -2 ̊C at 50 km altitude. The layer above the troposphere is the stratosphere, which is heated by the absorption of intense ultraviolet radiation from the sun(Figure 10.2). There is virtually no water vapor in the stratosphere, and it contains ozone, O3, and O atoms as the result of ultraviolet radiation acting upon stratospheric O2. Beyond the stratosphere are layers called the mesosphere and thermosphere, but they are relatively less important in the discussion of the atmosphere.