9.5: Greening of Water - Purification Before Use
- Page ID
- 285344
From the air the San Marcos/ Cazones River near the city of Poza Rica, Mexico, has a beautiful green color. But this river’s water is not what normally would be chosen as a water source (although by necessity it is so used), the reason being that its appearance denotes a major pollution problem because its green color indicates excessive growth of plants and algae (eutrophication) in the river, nourished by untreated sewage and other wastes discharged into it. This chapter discusses keeping water green, not in color but in terms of water quality.
For most uses water requires treatment. The processes used to treat water are generally similar for municipal, commercial, or industrial uses. This section addresses the major treatment processes applied to water supplies.
Municipal Water
The first steps in water treatment are usually physical, blowing air through it (aeration) and sedimentation to allow solids to settle from the water. Another benefit of water aeration is the oxidation of soluble Fe2+, a very undesirable pollutant that causes staining of bathroom fixtures and clothing, to solid Fe(OH)3, which settles from the water. A benefit of the formation of this gelatinous solid is that it acts as a coagulant that binds with colloidal impurities entrained in the water and causes them to settle. Another physical process to which municipal water is usually subjected is filtration to filter out solids. Filtration is usually over sand filters, but activated carbon, a form of carbon treated at high temperatures with steam or carbon dioxide to create pores and give it an enormous internal surface area, may be used as a filtration medium to remove organic substances.
Some water sources have too much dissolved Ca2+ (water hardness) that forms precipitates with soap and deposits solid CaCO3 in pipes. Normally the dissolved calcium is present as calcium bicarbonate in which the anion present with Ca2+ is bicarbonate, HCO3-. Curiously, the method for removing this calcium is to add more calcium in the form of lime, Ca(OH)2, a basic substance that converts the HCO3- to CO32- and removes the dissolved calcium as solid CaCO3 according to the following reaction:
\[\ce{Ca^{2+} (aq) + 2HCO_{3}^{-} (aq) + Ca^{2+} (aq) + 2OH^{-} (aq) \rightarrow 2CaCO3 (s) + 2H2O}\]
The final step in water purification is disinfection, commonly by the addition of elemental chlorine gas, which reacts with water,
\[\ce{Cl2 + H2O \rightarrow HOCl + H^{+} + Cl-}\]
to produce disinfectant HOCl that kills virus and bacteria in water. Hypochlorite salts of HOCl including NaOCl and Ca(OCl)2 can also act as disinfectants. Chlorine and hypochlorites can react with low concentrations of added ammonia to produce chloramines such as NH2Cl that remain in the water distribution system and maintain residual disinfection In the presence of residual organic matter such as humic substances, elemental chlorine may form potentially toxic chloroform, HCCl3, and a class of related substances commonly called trihalomethane compounds. To prevent these compounds from forming, a common practice is the use of chlorine dioxide, ClO2, which does not produce trihalomethanes. Chlorine dioxide is too dangerously reactive to move and too unstable to store so it is made on site by the reaction of sodium chlorite with elemental chlorine:
\[\ce{2NaClO2 (s) + Cl2 (g) \rightarrow 2ClO2 (g) + 2NaCl}\]
Therefore, potentially dangerous chlorine dioxide is prepared only when needed, in the quantities needed, where needed, which is in keeping with the best practice of green chemistry and technology.
Green Ozone
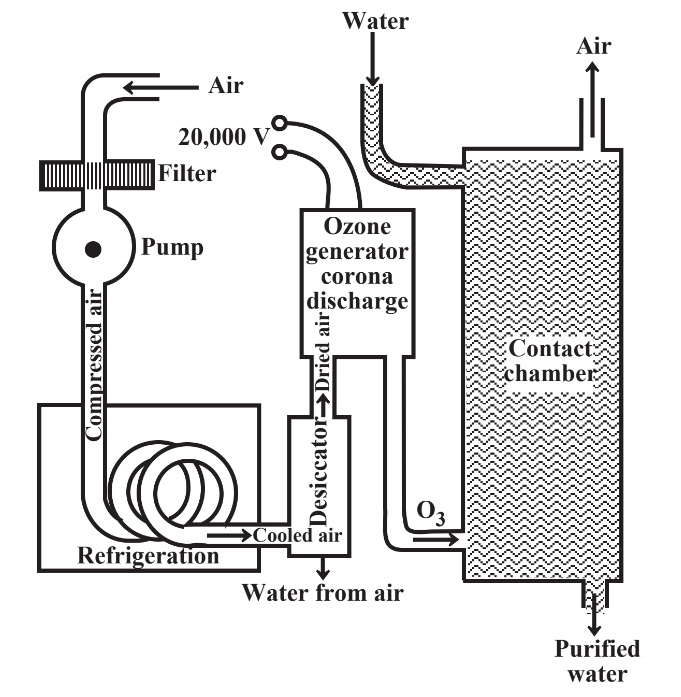
A greener alternative to chlorine-based water disinfectants in many respects is ozone, O3. Pumped into water, this form of oxygen kills pathogens without producing the undesirable byproducts made by chlorine and it is actually more effective than chlorine in killing viruses. Ozone is produced from oxygen in air by a high-voltage electrical discharge through dried air as illustrated in Figure 9.6. The lifetime of ozone in water is short, so a small amount of chlorine must usually be added to ozonated water to maintain disinfection in the water distribution system.
Disinfection of water by ozonation is a virtually ideal example of green chemical practice. The only raw material is universally available air, which is free. Ozone is produced only where it is needed as it is needed, without byproducts. The ozone does not persist in water, decomposing to elemental oxygen. There is very little likelihood of producing harmful disinfection byproducts with ozone.
Water for Commercial and Industrial Use
A wide range of water quality is required for water destined for commercial and industrial use and for economic reasons the water is treated only as needed for its intended application. Water used for cooling generally requires little treatment, the main requirement being that it is wet. At the other end of the scale water used in the semiconductor industry has to be hyperpure. Pathogens obviously must be removed for water employed in food processing and corrosive and scale-forming contaminants must be removed from boiler feedwater.
The same treatment processes described above for municipal water are applied to water destined for commercial and industrial use. Some other treatment steps commonly applied to commercial and industrial water supplies include the following:
• Addition of precipitants such as Na3PO4 to remove Ca2+ and prevent formation of CaCO3
• Addition of dispersants to prevent scale formation
• Adjustment of pH by addition of acid or base
• Disinfection to remove pathogens and addition of biocides to prevent microbial growths
• Addition of coagulants followed by filtration to remove suspended colloidal material
• Treatment with activated carbon to remove organics
• Deionization to remove salts
• Reverse osmosis to remove salts
For economic and conservation reasons significant amounts of water used for commercial and industrial applications are recycled as discussed in Section 9.7. Such water is often subjected to sequential use for applications that require successively lower quality, the last use before discharge requiring the lowest quality of water. In some cases water leaving a facility may be applied to grass or golf courses or used for irrigation.


