9.3: Water Chemistry
- Page ID
- 285342
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Some of the chemical and biochemical phenomena that occur in water are illustrated in Figure 9.4.2 Physically, the body of water shown is stratified during summer months with a warmer, less dense epilimnion floating over a colder, more dense hypolimnion, with little mixing between the two. The epilimnion is in contact with the atmosphere and has a significant content of dissolved oxygen. Therefore, oxidized inorganic species predominate in the epilimnion. The hypolimnion is anoxic because microorganisms consume all the dissolved O2 in it and it is not in contact with the atmosphere. Reduced inorganic species predominate in the hypolimnion. A major factor in the chemistry of this system is the biochemical photosynthetic production of organic matter represented {CH2O}. Organic matter is a biochemical reducing agent and when it sinks into the hypolimnion it is oxidized by microorganism-mediated processes that, for example, reduce NO3- and SO42- to NH4+ and H2S, respectively. Two important microbially-mediated oxidation-reduction reactions of{CH2O} are reaction with dissolved O2,
\[\ce{(CH2O) + O_{2} \rightarrow CO2 + H2O}\]
which depletes dissolved oxygen in water making the hypolimnion anoxic and methane fermentation,
\[\ce{2(CH2O) \rightarrow CH4 + CO2}\]
which produces combustible methane gas. The ability of {CH2O} to react with dissolved O2 is a measure of the potential of water to become depleted in the oxygen needed by fish and other aquatic organisms and is expressed as biochemical oxygen demand, BOD, an important water quality parameter.
The photosynthetic biochemical production of biomass results in some important chemical reactions. As illustrated in Figure 9.4, algae use dissolved HCO3- ion as a carbon source and in so doing produce carbonate ion, CO32-. Two additional reactions of carbonate ion are shown in Figure 9.4. One is its hydrolysis reaction with H2O molecules back to HCO3- with production of OH- ion. This makes the water basic, an important aquatic acid-base reaction. A second reaction of carbonate is that with dissolved Ca2+ to produce solid CaCO3, an important precipitation reaction in water that has been responsible for formation of large deposits of limestone.
All major oxidation-reduction reactions in natural water are carried out by microorganisms acting as catalysts and extracting energy released by the reactions. An example shown in Figure 9.4 is the biochemical reduction of SO42- to H2S. in which sulfate acts as an oxidizing agent to oxidize biomass ({CH2O}) in the absence of molecular O2. Taking place in the hypolimnion and sediments, this reaction is responsible for the foul odor of hydrogen sulfide in some bodies of water and swamps.
Another important phenomenon in natural water and wastewater is the formation of metal chelates in which metal ions are bound in two or more places by organic substances. Humic substances produced by the partial biodegradation of biomass are complex large organic molecules with numerous aromatic ring structures containing oxygen in functional groups including carboxyl (-CO2H) and phenolic hydroxyl (-OH). These groups can lose H+to produce negatively charged groups capable of bonding with metal ions as shown below for the chelation of Fe2+ ion:
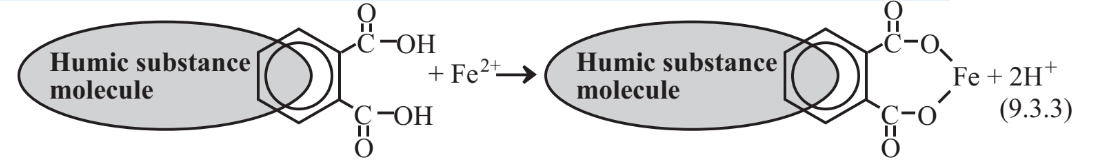
The most important humic substances in water are the lower-molecular-mass fulvic acids. These species tend to chelate Fe2+ ion producing a yellow material called gelbstoffe (German for “yellow stuff.)” Metal ions bound with fulvic acid are hard to remove from water and, since iron is a very undesirable water impurity, drastic measures such as destruction of the fulvic acid with chlorine may be required to remove the chelated iron.
Phase Interactions in Aquatic Chemistry
Figure 9.4 illustrates the process of exchange of dissolved solutes in water with sediments. Interactions between water and solid, gas, and other liquid phases are very important in aquatic chemistry. Aquatic biochemical processes involve exchange of materials between water solution and cells of microorganisms. For example, when photosynthesis occurs in water (Figure 9.4), dissolved HCO3- ion is transferred into a cell of floating photosynthetic phytoplankton for conversion to biomass. As a product of this reaction gaseous O2 is released from the cell, some of it dissolving in water and some floating to the top as O2 bubbles. As noted above, the CO32-ion generated as a byproduct of photosynthesis reacts with dissolved Ca2+ ion to produce solid CaCO3. The reverse process occurs when bacterially-produced dissolved carbon dioxide reacts with solid calcium carbonate.
\[\ce{CO2(aq) + H2O + CaCO3 (s) \rightarrow Ca^{2+} (aq) + 2HCO3^{-} (aq)}\]
to put calcium ion and bicarbonate ion into solution. Both of these species are important in water. The Ca2+ ion is responsible for water hardness, named for a tendency to form precipitates with soap anions that are useless for cleaning, and HCO3- is water alkalinity, the ability to neutralize acids.
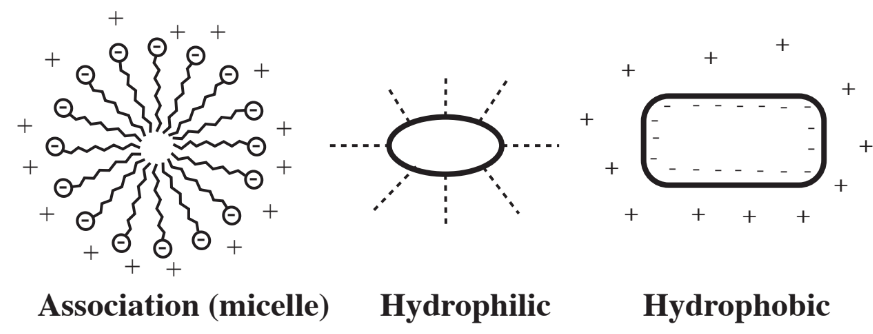
A particular kind of phase that interacts with water consists of colloidal particles, which are very small particles of the order of a micrometer in size that are suspended in water. There are three kinds of colloidal particles as shown in Figure 9.5. Many significant materials exist as colloidal particles in water including bacterial and algal cells, clay minerals, soap, and a variety of pollutants. The behavior of colloids is intermediate between that of true solutions and bulk materials such as those in sediments. This behavior is strongly influenced by the very high surface-to-volume ratio of colloids. Aggregation of colloidal particles is an important and often challenging aspect of water treatment, for example in the settling and separation of microorganisms involved in the biological treatment of wastewater.
Acid-Base Phenomena in Water
Natural water almost always contains acids capable of releasing H+ ion and may contain solutes that can accept H+ ion and thus act as bases. The most common acidic substance in water is dissolved CO2, which may enter water from the atmosphere or in higher concentrations as a product of the microbial decay of organic matter. Dissolved carbon dioxide produces H+ ion by the following reaction with water:
\[\ce{CO2 (aq) + H2O \leftrightarrows H+ + HCO3^{-}}\]
The double arrows denote that the reaction is reversible. Since carbon dioxide is a weak acid, the equilibrium of the reaction lies to the left as expressed by the following acid dissociation constant in which pKa1= -logKa1:
\[\K_{a1} = \frac{[H^{+}][HCO_{3}^{-}]}{[CO_{2}]} = 4.45 \times 10^{-7} \: \: \: pK_{a1} = 6.35\]
The HCO3- ion can also lose H+:
\[\ce{HCO3^{-} \leftrightarrows H+ + CO3^{2-}}\]
\[K_{a2} = \frac{[H^{+}][CO_{3}^{2-}]}{[HCO_{3}^{-}]} = 4.69 \times 10^{-11} \: \: \: pK_{a2} = 10.33\]
Otherwise pure water in equilibrium with air contains some dissolved CO2 from the atmosphere, which is 390 parts per million CO2 by volume. Solubility calculations can be used to show that the dissolved carbon dioxide concentration water, [CO2(aq)], in equilibrium with air is 1.276×10-5 M (moles/liter). When each CO2 reacts with H2O, one H+ and one HCO3- areproduced. Substitution into the equilibrium constant expression Equation 9.3.6 leads to [H+] =2.38×10-6 M, pH = 5.62, slightly more acidic than neutral pH 7. Therefore, rainwater is naturally slightly acidic. Natural water with a pH less than 5.62 likely contains pollutant strong acid, most commonly H2SO4 from acid rain.
Most water that has been in contact with the geosphere contains alkalinity, the ability to react with H+ ion and neutralize acidity. Generally alkalinity is due to the presence of HCO3- ion which undergoes the following reaction with H+ ion:
\[\ce{HCO3^{-} + H^{+} \rightarrow CO2 + H2O}\]
Alkalinity is normally introduced into water by the reaction of dissolved CO2 with CaCO3 mineral as shown in Reaction 9.3.4. Because of the presence of alkalinity, most natural waters such as those used to supply municipal water systems are slightly basic with a pH around 8 rather than being slightly acidic like rainwater.


