5.7: Oxidation-Reduction Reactions and Green Chemistry
- Page ID
- 285303
Many reactions, including some of those given in the preceding section, are oxidation-reduction reactions, frequently called redox reactions. This name derives from the long standing use of oxidation to describe the reaction of a substance with oxygen. Consider the following reaction of elemental calcium with elemental oxygen:
\[\ce{2Ca + O2 \rightarrow 2CaO}\]
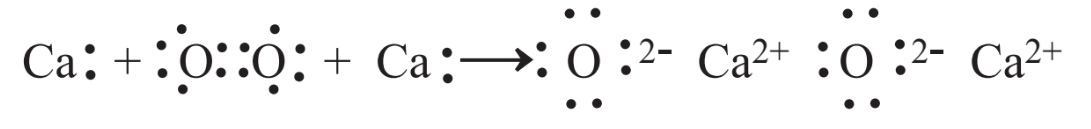
Combining with oxygen, Ca is oxidized. Whenever something is oxidized, something else has to be reduced. In this case, elemental oxygen is reduced to produce the oxide ion, O2-in CaO. It is seen from this reaction that the calcium atoms lose electrons when they are oxidized and the oxygen atoms gain electrons. This leads to another definition of oxidation-reduction reactions, which is that when a chemical species loses electrons in a chemical reaction it is oxidized and when a species gains electrons it is reduced.
Elemental hydrogen is commonly involved in oxidation-reduction. Whenever a chemical species reacts with elemental hydrogen, it is reduced. As an example, iron(II) oxide, FeO, can be reacted with elemental hydrogen,
\[\ce{FeO + H2 \rightarrow Fe + H2O}\]
In this case the Fe in FeO is reduced to iron metal and the hydrogen in elemental H2 is oxidized to H2O.
When elemental oxygen reacts to produce chemically combined oxygen, it is acting as an oxidizing agent and is reduced. And when elemental hydrogen reacts to produce chemically combined hydrogen, it acts as a reducing agent and is oxidized. Consider what happens when the opposite reactions occur. When chemically combined oxygen is released as elemental oxygen from a chemical reaction, the oxygen is oxidized. And when elemental hydrogen is released as the result of a chemical reaction, hydrogen is reduced. A good illustration of these definitions may be seen when a direct electrical current is passed between two metal electrodes through water made electrically conducting by dissolving in it a salt, such as Na2SO4 as shown in Figure 5.1. At the left electrode, electrons are pumped into the system reducing the chemically bound H in H2O to elemental H2. An electrode at which reduction occurs is called the cathode. At the other electrode, electrons are removed from the system, elemental O2 is released, and the oxygen in H2O is oxidized. An electrode at which oxidation occurs is called the anode.
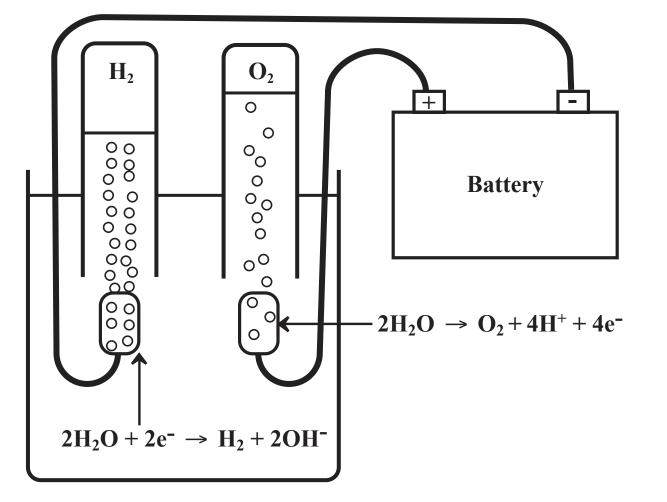
Figure 5.1. Electrolysis of water containing some dissolved salt to make it electrically conducting. At the left electrode(cathode) H in H2O is reduced by adding electrons releasing H2 gas. At the right electrode (anode) electrons are removed from chemically bound O in H2O releasing elemental O2 and the oxygen is oxidized.
The reaction shown above is an electrolysis reaction. It is very significant in the practice of green chemistry because it is a means of getting pure hydrogen and pure oxygen from water without the use of any other chemical reagents. For example, using a nonpolluting source of energy, such as wind power, elemental hydrogen can be generated for use in nonpolluting fuel cells (see Figure 3.2 and Chapter 16)
Oxidation-reduction reactions are very significant in energy conversion processes. An important example is photosynthesis,
\[\ce{6CO2 + 6H2O + h \nu \rightarrow C6H12O6 + 6O2}\]
in which solar energy (\(h \nu\)) from sunlight is used by plants to produce glucose sugar, C6H12O6, a high-energy compound that is used by organisms to provide energy for their metabolic needs. Since elemental oxygen is produced, oxygen is oxidized. Although it is not obvious based upon the discussion of oxidation-reduction so far, carbon is reduced; the carbon in the C6H12O6 product is reduced compared to the carbon in the CO2 reactant. The reverse of this reaction shown at the beginning of this chapter is
\[\ce{C6H12O6 + 6O2 \rightarrow 6CO2 + 6H2O + energy}\]
which occurs when organisms—including humans—utilize glucose sugar to produce energy. In this case, oxygen reacts, an obvious oxidation process. The oxygen is reduced and carbon is oxidized by the action of the elemental oxygen.
A very common oxidation-reduction reaction occurs when fossil fuels are burned to produce energy. One such reaction occurs when natural gas (methane, CH4) burns,
\[\ce{CH4 + 2O2 \rightarrow CO2 + 2H2O + energy}\]
to produce carbon dioxide and water, releasing energy. The burning of gasoline, diesel fuel, coal,wood, and even hydrogen gas are oxidation-reduction reactions in which carbon or hydrogen are oxidized by the action of oxygen yielding usable energy.
Oxidation-reduction reactions are the most important kinds of reactions considered in green chemistry. That is true in part because of the central role currently played by the oxidation of fossil fuels and other materials in producing energy needed for chemical processes. Furthermore, the most common raw material currently used for making plastics, synthetic fabrics, and other manufactured materials is petroleum hydrocarbon. There are many hydrocarbon compounds all containing chemically bound carbon and hydrogen. A typical such compound is ethane, C2H6. The hydrogen and carbon in a hydrocarbon are in the most chemically reduced form, but required raw materials often are partially oxidized hydrocarbons in which O atoms are bonded to the hydrocarbon (complete oxidation of a hydrocarbon yields CO2 and H2O). Ethanol, C2H6O, used in chemical synthesis and as an oxygenated additive to make gasoline burn more smoothly with emission of fewer air pollutants is a partially oxidized hydrocarbon.
Large quantities of materials and energy are expended in converting petroleum hydrocarbons to partially oxidized compounds used as raw materials. For example, ethanol can be made from ethane taken from petroleum and natural gas by a series of chemical reactions for which the net process is the following:
\[\ce{2C2H6 + O2 \rightarrow 2C2H6O}\]
This transformation requires relatively severe conditions and a net loss of energy. A greener alternative is to use glucose sugar produced by photosynthesis (Reaction 5.7.3) to grow yeasts that produce an ethanol product
\[\ce{C6H12O6 \rightarrow 2C2H6O + 2CO2}\]
a process that occurs under room temperature conditions. In addition to making ethanol, this fermentation process yields carbon dioxide in a concentrated form that can be used for carbonated beverages, supercritical carbon dioxide solvent, or pumped underground for tertiary petroleum recovery. The protein-rich yeast biomass produced in fermentation makes a good animal feed additive.


