5.3: Specific Replacements
- Page ID
- 112505
Alternative Reagent Strategies
Removal of Lactose
The opportunity to remove lactose through a separation process can be achieved by attaching glutaraldehyde (two terminal-ended aldehyde group below, left) to an aminated silica particle (image on right) which is then covalently linked to a galactosidase enzyme that can be used to remove lactose from milk products followed by coupling with galactosidase and reaction with milk.
Quaternization of Amines
Quaternary ammonium cations, quats, are positively-charged ions of the structure shown below:
The R group can be an alkyl group or an aryl group. Unlike ammonium (NH4 + ), quaternary ammonium cations are permanently charged, independent of pH.
Combinatorial Chemistry
Combinatorial chemistry is an approach to chemical synthesis that makes possible the preparation of a large number (e.g., millions) of compounds in a single sweep. These libraries can be mixtures, individual compounds, or computational structures.
Combinatorial chemistry can be used for small molecules, as well as for biomacromolecules such as peptides. Synthesis can quickly lead to large numbers of products. As an example, a molecule with three points of functionality or diversity (R1, R2, and R3) can generate structures, where NR# = the numbers of different substituents utilized. The basic principle of combinatorial chemistry is the preparation of large numbers of compounds that are then used to identify the useful components of the libraries.
Although combinatorial chemistry has been in play over the last 25 years, its roots go to the 1960s when a Rockefeller researcher, Bruce Merrifield, started investigating solid phase synthesis of peptides. It has had its biggest impact in the drug industry where researchers optimize the activity profile of a compound by creating a library of many different but related compounds.
Starch
Starch is an energy storage biomaterial generated from carbon dioxide and water during photosynthesis. Among the panoply of natural polymers, starch is of considerable interest because of its biodegradability, low cost, renewability, and biocompatibility. It is therefore considered a promising candidate for the sustainable development of new functional materials.
Starch is composed of two homopolymers of D-glucose: amylase, a virtually linear α-D- (1, 4’)- glucan, and branched amylopectin, with many α-1, 6’-linked branch points (Figure \(\PageIndex{4}\) ).
Starch is a polyol: it has therefore, two secondary hydroxyl groups at C-2 and C-3, and one primary hydroxyl at C-6 when it is not linked. It is also quite hydrophilic and can be oxidized and reduced for the formation of ethers and esters and many other functional molecules. Starch has various proportions of amylose and amylopectin from about 10–20% amylose and 80–90% amylopectin based on the source. It forms a helical structure and occurs naturally as discrete granules because the short- branched amylopectin chains can form helical structures to crystallize. It has a MW of 500-20K consisting of a-(1à4)-D-glucose units whose extended shape has a 7-22 nm hydrodynamic radius range. The helical form of the molecule usually forms a stiff left- handed single helix whose structure, not unlike DNA, is supported by H-bonding between O2 and the O6 atoms. Amylose can undergo syneresis (dehydration synthesis) between vicinal (neighboring) glucan residues to form the cyclodextrin cavity in which the interior maintains a hydrophobicity. Interestingly, it can form double-stranded crystallites that are resistant to amylase (the putative enzyme for its deconstruction), and H-bonding and solubility. The crowing macromolecule from starch, CD, is a veritable green chemist’s dream owing to a number of wonderful properties. Shown below is the toroidal structure of CD and dimensions:
How do Cyclodextrins Work?? https://www.youtube.com/watch?v=UaEes6cJu7k (How do Cyclodextrins work? (1980). Cyclolab.)
Specifically, it is a cyclic oligomer of a-D-glucopyranose held together by glycosidic bonds very much like what is seen in all polysaccharides. It was discovered in 1891 by Villiers who synthesized by the enzymatic conversion of amylose followed by a selective precipitation. The precipitation can be done with several solvents, albeit organic in nature and not sustainable, but with varying degrees of yields. Note that we look at three forms of the CDs as shown in the graph below:
| Precipitating Agent | Yield (%) | |
| a-CD | 1-decanol | 40 |
| b-CD | toluene | 50-60 |
| g-CD | Cyclohexadec-8-en-1-ol | 40-50 |
What is remarkable about the CDs is their ability to form host/guest inclusion complexes owing to their unique HLB (Hydrophilic Lipophilic Balance) criteria. These are quite literally molecularscale reactors that can do many things. In the context of biomedical applications, the focus for our particular discussion, the following are among their benefits.
- To increase aqueous solubility of drugs
- To increase chemical stability of drugs
- To enhance drug delivery to and through biological membranes
- To increase physical stability of drugs
- To convert liquid drugs to microcrystalline powders
- To prevent drug-drug and drug-excipient interactions
- To reduce local irritation after topical or oral administration
- To prevent drug absorption into skin or after oral administration
The nanoreactors have an uncanny capacity to encapsulate and/or do chemistry with a number of chemicals that are hydrophobic or low polarity. Shown in Figure 5-9 is a cartoon that depicts the hydrolytic action of CD with a phosphate molecule.
Figure \(\PageIndex{6}\): A simplified representation of the encapsulating and chemical reactivity behavior of a CD macromolecule for phosphate hydrolysis. https://commons.wikimedia.org/wiki/F...ease_Mimic.png
CD can by virtue of the varieties in its sizes accommodate relatively small to large guest molecules. Shown below in Figure 5-10 is a pictorial description of a 1:2 guest:host complex that engages in photophysical behavior.
The system is engaging in an energy transfer from the peripheral boron- containing molecules to the encapsulated porphyrin molecules which releases a photon of light upon resonant energy transfer (645 nm) from the peripheral molecules.
Phase Solubility Diagrams
Phase solubility studies are carried out in aqueous systems at different temperatures to calculate stability (equilibrium) constants, (Kc) and thermodynamic values for the formation of inclusion complexes. It is known that if phase solubility diagrams show that the solubility of a guest molecule increases linearly along with the concentration of CD, then they can be considered as AL-type phase diagrams [1], suggesting the formation of 1:1 complexes, which are the most common and best understood of these types of interactions. When the phase solubility data are collected at different temperatures, we can obtain valuable additional information such as the thermodynamic parameters for the formation of the complex.
The integrated form of the Van’t Hoff Equation allows for the calculation of the enthalpy (DH) and entropy changes (DS) depending on the variations of the stability constants with temperature [2]:
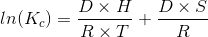
The Kc can also be expressed as the following relationship:
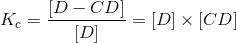
that can be pictorially described as:
in which there is a complexation with D (the substrate or drug in this case) and the CD. The above represents an AL-type complex which is first order with respect to the CD and the drug (D).
A-type phase-solubility profiles are defined by the solubility of the substrate (drug) increasing with increasing ligand (cyclodextrin) concentration. When the complex is first order relative to the ligand and first or higher order with respect to substrate then AL-type phase-solubility profiles are obtained. If the complex is first order with respect to the substrate but second or higher order with respect to ligand, AP-types are obtained. AN-type phase-solubility profiles are difficult to interpret. B-type phase- solubility profiles indicate formation of complexes with limited solubility in the aqueous medium. The water-soluble cyclodextrin derivatives form A-type phase- solubility profiles while less soluble cyclodextrins form B-type profiles. A number of drug/cyclodextrin complexes form inclusion complexes, butcyclodextrins form non- inclusion complexes and aggregates that dissolve drugs through micelle-like structures. The phase-solubility profiles do not verify inclusion complexes, but only describe how increasing cyclodextrin concentration influences drug solubility. As we already discussed, the most common type of cyclodextrin complexes are the 1:1 where one drug molecule (D) forms a complex with one cyclodextrin molecule (CD). In an AL-type phasesolubility diagram where the slope is less than unity, the stability constant (K1:1) of the complex can be calculated from the slope and the intrinsic solubility (S0) of the drug in the aqueous media (i.e., drug solubility when no cyclodextrin is present):
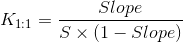
The value of K1:1 is typically 50 < 2000 M-1 with a mean value of 129, 490, and 355 M-1 for α-, β- and γ- cyclodextrin, respectively [3].


