2.28: High Performance Liquid Chromatography (HPLC)
- Page ID
- 120668
RELATED READINGS: Pages 139-135.
OBJECTIVES
Upon completion of this exercise, appropriate discussion, and related
readings, the student will be able to:
- Interpret the pattern of a high performance liquid chromatography chromatogram.
- Calculate certain parameters of a chromatogram including resolution and capacity factor.
- Calculate the concentration of an unknown by the external standardization technique.
PRINCIPLE
Standardization of an HPLC assay is usually performed by proportional analysis, comparing the peak height (PH) of the analyte in the unknown to the peak height of a known amount (C) of standard:
\[\frac{C_{standard}}{PH_{standard}} = \frac{C_{unknown}}{PH_{unknown}}\]
This technique is also called external standardization. However, when a sample
purification step is used, the recovery of the extracted analyte from the sample is usually variable. The above proportional calculation is only valid if one assumes virtually complete recovery. Since this is not often true, two techniques are used to correct for variable recovery. These are the method of standard additions and the method of internal standardization (the latter technique will be discussed in Experiment # 29).
The method of standard addition allows one to estimate the recovery of the analyte during sample processing. This method requires 2 aliquots of the specimen. One aliquot is analyzed without any modifications while the second aliquot is analyzed following the addition of a known amount (spike) of the standard. The pure standard is analyzed by direct HPLC analysis, that is, without any extraction. The first step in the calculation is to determine the recovery of the standard added to the second aliquot.
PHS = peak height (mm) of standard
PHx = peak height (mm) of analyte in unspiked aliquot of unknown
PHsp = peak height (mm) of analyte in spiked aliquot of unknown
Cs = concentration of standard
Cx = concentration of analyte in unknown
Csp = final concentration of standard added to second aliquot of unknown. The measured Csp is compared to the actual estimate % recovery.
\[\frac{PH_{sp} - PH_{x}}{PH_{s}} = \frac{(C_{sp} + C_{x}) - C_{x}}{C_{s}} = \frac{C_{sp}\; (measured)}{C_{s}}\]
\[C_{sp}\; (measured) = \frac{(PH_{sp} - PH_{x}) \times C_{s}}{PH_{s}}\]
\[\frac{C_{sp}\; (measured)}{C_{sp}\; (actual)} \times 100 \% = \%\; \text{recovery of spike}\]
Once the % recovery from the matrix of the sample is determined, the concentration of the analyte in the unknown is calculated by proportional analysis and corrected for the % recovery:
\[\frac{C_{x}}{C_{s}} = \frac{PH_{x}}{PH_{s}}\]
\[C_{x} = \frac{PH_{x} (C_{s})}{PH_{s}} \times \frac{100 \%}{\%\; recovery}\]
When developing an HPLC method it is often useful to determine parameters defining the chromatographic system. These parameters are also useful in monitoring system performance. Frequently calculated parameters include the peak resolution (R) and the capacity factor (k). The equations for calculating these parameters, using just the elution times (t) or elution volumes (v) are described on pp. 112-117.
MATERIALS
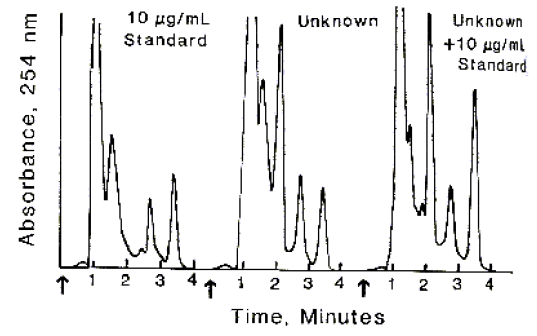
Sample chromatograms are found at the end of this exercise. The student will need a millimeter ruler and calculator. The sample chromatogram resulted from an
assay for serum theophylline performed as follows.
PROCEDURE
- Pipet two 0.10 mL aliquots of pooled serum into two 12 x 75 mm glass test tubes, labeled “spike” and “unspiked”, respectively.
- To the test tube labeled “unspiked”, add 0.10 mL of acetonitrile. To the test tube labeled “spike”, add 0.10 mL of theophylline standard.
- Mix by vortexing, and centrifuge for 2-5 minutes at 800 x g to pellet precipitated protein. Save supernatants for HPLC analysis.
- During centrifugation step, prepare working theophylline standard by mixing 0.10 mL of distilled water with 0.10 mL of 10 mg/L theophylline solution.
- Inject 10 mL of each supernatant and 10 mL of working theophylline standard into HPLC system for analysis. The chromatograms are shown on page
- Record time of elution of theophylline peak for standard and each supernatant. Measure the peak height (in mm) with a ruler.
| DATA SHEET, EXERCISE #28 |
NAME: ___________ DATE: ___________ |
RESULTS
| Elution time (min) Theophylline | Peak Height (mm) | Final Theophylline Concentration | |
|---|---|---|---|
| Working standard | |||
| Unspiked aliquot | |||
| Spiked aliquot |
CALCULATIONS
Calculation of Percent Recovery.
Cs = 10 mg/L,
Csp (measured) = __________mg/L
% recovery = [Csp (measured)]/10mg/L x 100% = \(\dfrac{\qquad}{10\; mg/L}\) x 100% = _____%
Calculation of theophylline concentration in unknown.
Cx = __________ x 100% = __________mg/L
Calculation of Capacity Factor (k').
Calculation of Peak Resolution (peaks at approximately 2.7 and 3.5 min.)
Discussion Questions
- Why is it essential to measure the retention time for each peak?
- What relationship does the elution time of an analyte have with the elution volume?
- Would the results differ if peak areas were used rather than peak heights?