2.26: Extraction Technique
- Page ID
- 120631
RELATED READINGS: Pages 125-126.
OBJECTIVES
Upon completion of this exercise, appropriate discussion, and related readings the student will be able to:
- Understand the techniques of liquid—liquid extraction.
- Purify and concentrate an analyte.
PRINCIPLE
There are a number of reasons why some type of extraction step is needed for an analytical method. These include: removing sample material that can destroy a component of the analytical system, (such as protein, that can “foul” an HPLC column), increasing the specificity of the assay by removing interfering compounds, placing the analyte in a medium that will allow a derivatization reaction, and concentrating the analyte for enhanced sensitivity.
Thus, in all cases, extraction techniques remove the analyte from its initial sample matrix. Extraction techniuqes can include: protein precipitation, column chromatography (ion-exchange, neutral resins, etc.), and liquid-liquid extraction. The latter technique is the most frequently used because of its simplicity. The analyte is typically extracted into a volatile organic solvent, allowing the analyte to be concentrated by evaporation of the organic solvent.
In this exercise, bilirubin in a serum sample will be extracted into chloroform and the amount extracted determined by spectrophotometric analysis. This extraction technique is often used to measure bilirubin in amniotic fluid because the colorimetric methods (Malloy-Evelyn or Jedrassik-Grof) lack sensitivity and direct spectrophotometric analysis of the amniotic fluid is subject to interference and lacks specificity.
MATERIALS
- Serum pool containing a known elevated amount of bilirubin
- Spectral-grade chloroform
- Spectrophotometer
- Normal saline
- Glass-topped, 50 mL centrifuge tubes (aluminum foil-wrapped)
- Centrifuge
PROCEDURE
- Dilute 3 mL of the serum pool 1:5 with normal saline (0.85%, 0.15M NaCl).
- In duplicate, pipet 5 mL of the diluted serum into 50 mL glass stoppered centrifuge tubes.
- Add 10 mL of chloroform to each centrifuge tube.
- Stopper the centrifuge tubes, and extract by vortexing or shaking the tubes for 30 seconds. Centrifuge at low speeds for 3-5 minutes to separate the two phases. If a protein mesh persists in the chloroform (lower) layer after centrifugation, break it up with a glass stirring rod, and recentrifuge.
- Remove as much of the upper aqueous layer as possible with a Pasteur pipet and discard.
- Use another Pasteur pipet to carefully transfer the chloroform layers to two aluminum foil covered 16 mm x 150 mm test tubes. Avoid contamination of the remaining aqueous phase by passing the tip of the pipet into the lower layer before aspirating the fluid. Cover, and place in dark.
- Take one of the test tubes, and evaporate the sample to dryness by passing a stream of air or N2 gas over the surface of the liquid. The evaporation can be hastened by placing the test tube in a 37°C water bath. The evaporation must be performed in a hood or ventilated work space, since chloroform is toxic.
- Reconstitute the residue in 1 mL of chloroform. Measure the absorbance spectra of both chloroform solutions from 350 nm to 550 nm at 25 nm increments. Record the absorbance readings on the data sheet.
- Measure the absorbance spectrum of the diluted serum at the same wavelengths used in step #8. Record on the data sheet.
- Plot all the spectra on the graph paper provided on the data sheet.
Discussion Questions
- Why are the centrifuge tubes and glass test tubes covered with aluminum foil?
- Are the spectra of the 3 solutions (diluted serum, and 2 chloroform extracts) the same? If not, can you explain the differences?
- Which solution gave the highest absorbance at 450 nm? Do these absorbancies acurately reflect the actual bilirubin concentration in each solution? If not, why not?
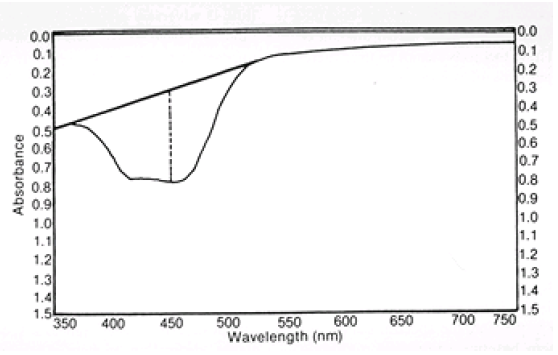
- Which of the solutions yielded a spectra most closely resembling that depicted in the figure below?
Amniotic fluid scan showing the method for determining absorbance at 450 nm. Arbitrary (base) line drawn from 375 to 525 nm shows where scan would have traced if no bilirubin pigment were present. Broken line indicates the absorbance recorded (O.D. when ordinate is in optical density units).
| DATA SHEET, EXERCISE #26 |
NAME: ___________ DATE: ___________ |
RESULTS
Absorbance Measurements:
| Wavelength (nm) | Diluted Serum | 10 mL Chloroform | 1 mL Chloroform |
|---|---|---|---|
| 350 | |||
| 375 | |||
| 400 | |||
| 425 | |||
| 450 | |||
| 475 | |||
| 500 | |||
| 525 | |||
| 550 |
Plot each spectral curve on the semi-logarithmic graph paper provided on the next page. Use a different colored pen for each sample.
Using the tangent method described on page 433, determine the absorbance at 450 nm, corrected for baseline.
Use Beer’s Law, the absorbance at 450 nm by the tangent method, the molecular weight of bilirubin (569 g/mole), and the extinction coefficient for bilirubin of 60,700 L •mo1-1 • cm-1 to calculate the concentration of bilirubin in each sample.
CALCULATIONS
\[A = \epsilon c \ell\]
| Sample | A450 from Tangent Method |
Concentration of Bilirubin by Absorbance Calculation |
Calculated Bilirubin in Diluted Serum |
||
|---|---|---|---|---|---|
| umol/L | mg/L | umol/L | mg/L | ||
| Diluted serum | |||||
| 10 mL Chloroform | |||||
| 1 mL Chloroform | |||||
The actual bilirubin concentration in the control sample is __________mg/L
Assuming 100% extraction of the serum bilirubin into chloroform, calculate the
concentration of bilirubin in the diluted serum sample from the concentration of bilirubin in the chloroform extracts using the following formula:
\[[Bili]serum (vol. \; extracted) = [Bili]CHCl_{3} \; (vol. \; of\; CHCl_{3})\]
where: [Bili]serum = calculated bilirubin concentration in diluted serum
[Bili]CHCl3 = measured bilirubin concentration in chloroform extracts
Record results above, and compare to the expected value based on a five-fold dilution of the quality control sample’s known value.