5: The Composition of Potassium Chlorate (Experiment)
- Page ID
- 95810
- To experimentally determine the mass percent of oxygen in the compound potassium chlorate (\(\ce{KClO3}\)) via the thermal decomposition of a sample of potassium chlorate.
- To qualitatively demonstrate that the residue resulting from the decomposition of potassium chlorate is potassium chloride.
All compounds consist of elements chemically combined in fixed proportions – they obey the Law of Constant Composition. One way to express the proportion each of element in a compound is as a percentage by mass, or mass percent.
In Part A of this lab, a sample of potassium chlorate will be experimentally analyzed in order to determine the mass percent of elemental oxygen present in it. To do this, the potassium chlorate must be heated to temperatures greater 400 °C, causing it to thermally decompose into potassium chloride and free oxygen:
\[\ce{2KClO3 (s) ->[heat] 2KCl(s) + 3O2 (g)}\]
\[\text{Potassium Chlorate} \ce{->} \text{Potassium Chloride} + \text{Oxygen}\]
Students will perform a quantitative analysis of the reactants and products of this reaction, measuring the initial mass of solid potassium chlorate used (before heating), and the mass of the solid potassium chloride product, or residue, remaining after heating. Applying the Law of Mass Conservation, the difference in these measured masses is the mass of oxygen released (from the original potassium chlorate sample). From this data, the experimental mass percent of oxygen in potassium chlorate will be determined:
\[\text{Mass Percent of Oxygen (experimental)} = \frac{ \text{Mass of Oxygen Released}}{ \text{Mass of Potassium Chlorate Used}} \times 100\]
Mass percentages of elements in compounds can also be theoretically calculated using molar masses, along with the known chemical formula of the compound. Thus, the theoretical mass percent of oxygen in potassium chlorate would be calculated using the expression:
\[ \text{Mass Percent of Oxygen (theoretical)} = \frac{3 \times \text{(Molar Mass of O)}}{ \text{Molar Mass of } \ce{KClO3}} \times 100\]
Students can therefore evaluate their accuracy in this experiment by comparing their experimental results to the true theoretical value, and by calculating their percent error.
In Part B of this lab, the residue left after heating will be qualitatively analyzed in order to demonstrate that it is chemically different from the initial potassium chlorate sample. Specifically, the residue will be tested for the presence of chloride ions by the addition of nitric acid and aqueous silver nitrate. A positive test is indicated by the formation of a white precipitate. The actual identity of the residue will then be conclusively verified by comparing this result to those obtained for identical tests on known samples of potassium chlorate and potassium chloride.
Procedure
Materials and Equipment
Solid potassium chlorate (\(\ce{KClO3}\)), solid potassium chloride (\(\ce{KCl}\)), 6M nitric acid (\(\ce{HNO3}\)), 0.1M silver nitrate (\(\ce{AgNO3}\)), two crucibles with lids, stand and ring clamp, clay triangle, crucible tongs, Bunsen burner, three medium-sized test tubes, test tube rack, stirring rod, and an electronic balance.
Be especially careful when using the Bunsen burner and handling hot equipment. Remember that most items look exactly the same whether they are hot or cold. Heat the potassium chlorate sample slowly to avoid any splattering. Be aware that silver nitrate may stain the skin and nitric acid may burn the skin. If a spill of either chemical occurs, rinse under running water and report the accident to your instructor. Nitric acid spills may also be neutralized using the sodium bicarbonate solution by the sinks.
Part A: Mass Percent of Oxygen in Potassium Chlorate
The following steps should be carried out for two separate samples of potassium chlorate.
- Clean both crucibles and their lids (obtained from the stockroom) by thoroughly rinsing with distilled water then drying as completely as possible with a paper towel.
- Weigh the first crucible and lid on an electronic balance and record this mass on your report form.
- Add approximately 1 gram of potassium chlorate to the crucible. Do not do this over the balance! Then weigh and record the mass of the crucible, lid and potassium chlorate sample.
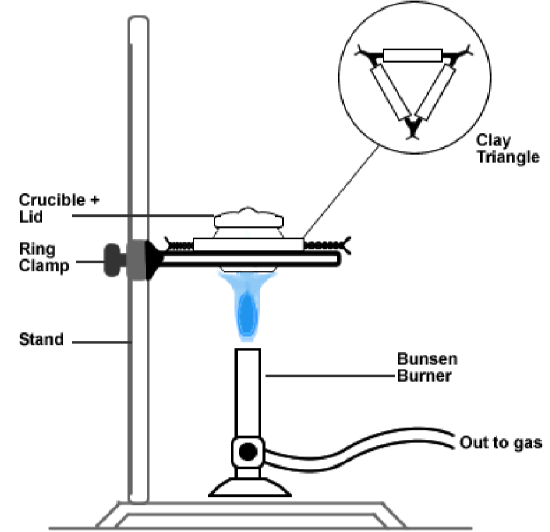
- Fetch a stand and ring clamp from the back of the lab. As shown in the figure and photo on the following page, place your clay triangle on the ring, and then place the crucible containing the sample onto the triangle. Cover the crucible with the lid.
- Using a Bunsen burner, heat the crucible and sample for a total of 12 minutes. Be sure that the crucible is covered, and that that the top of the flame is touching the bottom of the crucible.
- For the first 6 minutes, the sample should be gently heated by adjusting the Bunsen burner flame to a low-moderate temperature. Note that heating the sample too intensely at this point could cause loss of the sample via splattering, and could crack the crucible.
- For the last 6 minutes, the sample should be strongly heated by adjusting the Bunsen burner flame to a high temperature.
- Allow the crucible to cool to room temperature. Then weigh and record the mass of the crucible, lid, plus the residue that remains. Note that the weight of your sample is expected to decrease by at least 30 % of its original mass (~ 0.3 g).
- Now heat the sample a second time for an additional 6 minutes using a high temperature flame. Then, once again, allow it to cool to room temperature. Weigh the cooled crucible, lid and sample after this second heating and record the mass. If this mass is within 0.050 grams of your mass measurement after the first heating (see step 6), no further heating is necessary and you may begin Part B. Do not dispose of the residue as you will need it for Part B.
- If the sample from step 7 is not within 0.050 grams of the mass from step 6, heat again for a third time, cool and record the mass.
- Repeat all steps for your second crucible and second sample of potassium chlorate.
- Analysis: For each sample analyzed, obtain the mass of potassium chlorate before heating, the mass of \(\ce{KCl}\) residue after heating, and the mass of oxygen released. Use the residue mass after the final heating for these calculations. Then using these values, determine the experimental mass percent of oxygen in potassium chlorate (see Theory Section for equation required).
Part B: Qualitative Analysis of Residue
- Place three medium-sized test tubes in the test tube rack. The test tubes should be thoroughly cleaned and rinsed with distilled water. Label them tube #1, tube #2 and tube # 3. Continue to use only distilled water for the rest of Part B.
- To tube #1 add a pea-sized amount of potassium chlorate. Then fill tube #1 half-way with distilled water and mix with a stirring rod until the solid dissolves. If the solid does not dissolve completely, decant the clear part of the solution into a clean test tube, labeling this #1 instead.
- To tube #2 add a pea-sized amount of potassium chloride. Then fill tube #2 half-way with distilled water and mix until the solid dissolves. Be sure to rinse your stirring rod with distilled water first or you will contaminate your sample.
- Add some distilled water to your crucible and residue. The residue should dissolve. Then pour the resulting solution into tube #3. Repeat the process until tube #3 is half-filled (like tubes 1 and 2).
- To each of the tubes #1-3, add 6 drops of nitric acid immediately followed by 6 drops of silver nitrate solution. Then record your observations on the report form. Discard all test tube waste in the container provided.
- Analysis: Based on your observations for these tests (and any others observations made), what evidence do you have that the residue in your crucible is really potassium chloride?
Pre-laboratory Assignment: The Composition of Potassium Chlorate
- In Part A of this lab, you will analyze a sample of potassium chlorate to determine the mass percent of oxygen present in it. To perform the analysis, you will decompose the potassium chlorate by heating it. Write the word equation and the balanced formula equation for this decomposition reaction.
- Word Equation:
- Formula Equation:
- The potassium chlorate sample will be heated in a specialized "container".
- What is this container called?
- Will this container be covered or uncovered while heating?
- You will have to heat your sample of potassium chlorate at least twice.
- How long must the sample be heated the first time (total)?
- How long must the sample be heated the second time?
- A residue of potassium chloride will be left in the "container" after the heating is completed. Do you expect it weigh more than, less than or the same as the original potassium chlorate sample? Why?
- In Part A you will be performing several mass measurements. What are two precautions you must observe when using the electronic balance?
- In Part B of this lab, you will analyze the residue in left the "container" in order to experimentally verify its identity. To do this, you will need three test tubes. Potassium chlorate is added to tube #1, potassium chloride to tube #2, and the residue to tube #3. These solids are all dissolved in distilled water.
- What two chemicals will then be added to each of these substances to test them?
- What will you observe if you obtain a positive test for chloride ions?
Lab Report: The Composition of Potassium Chlorate
Part A: Mass Percent of Oxygen in Potassium Chlorate
Experimental Data
|
Sample 1 |
Sample 2 |
|
|---|---|---|
|
Mass of crucible + lid |
||
|
Mass of crucible, lid + \(\ce{KClO3}\) |
||
|
Mass of crucible, lid + residue after 1st heating |
||
|
Mass of crucible, lid + residue after 2nd heating |
||
|
Mass of crucible, lid + residue after 3rd heating |
Data Analysis
Use your data to determine the experimental mass percent of oxygen in \(\ce{KClO3}\). Show your work clearly for each step in the table below.
|
Sample 1 |
Sample 2 |
|
|---|---|---|
|
Mass of original \(\ce{KClO3}\) sample |
||
|
Mass of \(\ce{KCl}\) residue |
||
|
Mass of Oxygen released |
||
|
Mass Percent of Oxygen in \(\ce{KClO3}\) |
||
|
Average Mass Percent Oxygen |
||
Using molar masses along with the known formula of potassium chlorate, calculate the theoretical mass percent of oxygen in \(\ce{KClO3}\). Show your work clearly.
Calculate the percent error between your average experimental value and theoretical value for the mass percent of oxygen in \(\ce{KClO3}\). Show your work clearly.
Part B: Qualitative Examination of Residue
Observations and Analysis
|
Tube |
Observations (after the addition of both nitric acid and silver nitrate) |
|---|---|
|
#1: Potassium Chlorate |
|
|
#2: Potassium Chloride |
|
|
#3: Residue from Crucible |
Explain how your observations in the table above verify that the residue in your crucible after heating is potassium chloride.
Are there any other observations that you have made during this experiment (not those in the table above) that would suggest that the potassium chlorate was converted to a new substance upon heating?
Questions
- Was your average experimental mass percent of oxygen in potassium chlorate higher or lower than the theoretical value (circle one)? Higher/Lower
Which of the following sources of error could be used to explain this discrepancy (circle one)?
- The potassium chlorate sample was not heated strongly or long enough.
- Some of the potassium chloride product splattered out of the crucible during the heating process.
Explain your choice. Your response should include an analysis of the calculations you performed with your raw data to obtain your experimental % of oxygen.
- Suppose the stockroom made a mistake and gave you a mixture of potassium chlorate and potassium chlorite. Upon analysis of this mixture, would you obtain a larger or smaller mass percent of oxygen than you would for an equal mass of pure sample of potassium chlorate (circle one)? Larger Smaller
Explain your choice. Your response should include an analysis of the formulas of the compounds involved.
- Show your calculations clearly. Suppose you are provided with a 36.55 g sample of potassium chlorate.
- What mass of oxygen should theoretically be released upon heating?
- What mass of potassium chloride residue should theoretically be left over after heating?



