Chemistry in Sports, Physiology, and Health
- Page ID
- 50671
Chemistry has been defined as the science that is concerned with the composition, properties, and structure of matter and with the ways in which substances can change from one form to another. But this definition is too broad to be useful. Chemistry isn't the only science that deals with the composition and transformations of matter. Matter is also composed of foods, which may be transformed efficiently by digestion and other body processes into muscle or fat and energy. These are normally considered the subject of fields like nutrition, sports medicine, or exercise physiology. Chemists are unique because they have the potential to understand or explain everything on a molecular scale, and apply their knowledge to the subjects studied by nutritionists or exercise physiologists. This can be in terms of the properties of just over 100 kinds of atoms found in all matter, and the amazing variety of molecules that are created by forming and breaking bonds between atoms. So chemistry is defined by its approach, not its subject matter. Chemistry explains or understands any subject in terms of the properties of atoms and molecules.
Chemistry provides a unique perspective that complements many areas in sports and health.
Exercise physiologists use VOmax to determine the effectiveness of training for elite athletes, based on chemists' "gas laws" which show oxygen is a limiting reactant in metabolism. Chemical thermodynamics allows physiologists to predict the amount of energy that a body can produce based on VOmax, while helping dieters understand the energy value of foods.
NASCAR Drivers may owe their lives to chemistry. The driver of the vehicle in the composite photograph of a crash shown here walked away from the vehicle [1]

What saved his life was foam side panels made of Impaxx foam:
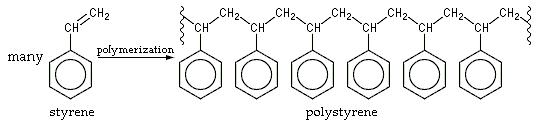
Chemistry is involved because knowledge of how styrene molecules bond to make polystyrene, an addition polymer, must be applied to make the material, as shown in the following diagram:[2]:
Physics is also involved, because the physical (nonmolecular) structure is what distinguishes Impaxx; unlike the open cell polystyrene of coffee cups that contains connected bubbles of gas, Impaxx has closed cells which prevent thematerial from collapsing under impact. That would be considered a physical change, as opposed to the chemical change that occurs when styrene polymerizes.
From ChemPRIME: 1.0: Prelude to Chemistry
References
- ↑ "Materials for the Modern Gladiator", Chemical & Engineering News, February 2, 2009; http://pubs.acs.org/cen/coverstory/87/8705cover.html
- ↑ en.Wikipedia.org/wiki/Polystyrene
Contributors and Attributions
Ed Vitz (Kutztown University), John W. Moore (UW-Madison), Justin Shorb (Hope College), Xavier Prat-Resina (University of Minnesota Rochester), Tim Wendorff, and Adam Hahn.


