Chemistry in Everyday Life
- Page ID
- 50669
Chemistry has been defined as the science that is concerned with the composition, properties, and structure of matter and with the ways in which substances can change from one form to another. But this definition is too broad to be useful. Chemistry isn't the only science that deals with the composition and transformations of matter. Matter is also composed of complex materials like animal hides which are transformed by craftspeople into beautiful leatherwork, or wood which is transformed into furniture, or cotton which is transformed into different grades of cloth. These are normally considered the subject of materials scientists or even consumer scientists. Chemists are unique because they understand or explain everything, even the subjects of importance to consumers in everyday life, in terms of the properties of just over 100 kinds of atoms found in all matter, and the amazing variety of molecules that are created by forming and breaking bonds between atoms. So chemistry is defined by its approach, not its subject matter. Chemistry explains or understands any subject in terms of the properties of atoms and molecules.
Chemistry can help craftspeople create more functional or interesting objects, or help consumers evaluate fraudulent or unsafe products. Has a cashier ever used a yellow pen to check if your paper money is counterfeit? The yellow marker is iodine (I2, combined with I- to make I3-), and if it turns blue/black, this indicates a counterfeit bill. The reaction is the classic iodine test for starch. When iodine enters the helical starch molecule as shown in the figure, it turns blue/black.
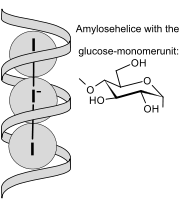
The ribbon-like starch molecule is made up of glucose molecules, shown in the figure, linked together. But to understand why this detects counterfeit money, we need to know more about paper.
"What's That Stuff" [2] is a regular feature of an American Chemical Society publication that gives examples of how chemistry is used to enrich everyday life. It tells us that Paper [3] is made by soaking plant fibers in water, then matting, pressing, and drying them. American paper money is made from a mix of cotton and cotton gin trash (75%) and flax waste (25%). Ordinary paper uses wood fibers which need to be bleached and starched to have the same quality surface, so they turn black when marked with the cashier's pen, while marks remain yellow on legitimate bills.
What holds paper together? Papermaking is an old art, dating from ancient Egypt where papyrus reeds from the Nile were used. It's a common hobby or profession that traditionally uses a process called retting to remove all but the cellulose from plant fiber. The cellulose is beaten in a Hollander to produce wet pulp. When it's pressed, the cellulose molecules form intermolecular bonds between the oppositely charged hydrogen and oxygen atoms shown in the figure.
in "natural foods" are no different than synthetic molecules made in the lab; they are both "chemicals" when understood in terms of their molecular structure or properties. When people speak of "chemical free" products, they usually mean that the material is found in nature and so was not designed by a chemist, even though the molecules designed by chemists are identical. Chemists often say 'everything is a chemical" because it is possible to understand everything in terms of molecular properties.
From ChemPRIME: 1.0: Prelude to Chemistry
References
Contributors and Attributions
Ed Vitz (Kutztown University), John W. Moore (UW-Madison), Justin Shorb (Hope College), Xavier Prat-Resina (University of Minnesota Rochester), Tim Wendorff, and Adam Hahn.


