Organic Nitrogen Compounds: Amines
- Page ID
- 50833
Organic Nitrogen Compounds and the Environment
Since nitrogen comprises 78% of the earth’s atmosphere by volume, it is not surprising that it would be an important component in many diverse organic molecules. With an electronegativity of 3.04, nitrogen forms stable covalent bonds with carbon, oxygen and hydrogen. The characteristic functional groups formed by nitrogen are typically active and therefore display a wide range of biological, environmental and industrial functions. One of the largest classes of organic nitrogen containing molecules is the amines. They are compounds which can be thought of as derivatives of ammonia, NH3. Amines are typically identified by the number of alkyl (carbon) groups attached to the nitrogen atom. In the examples below, methylamine is a primary amine since there is only one alkyl group attached to the nitrogen atom, dimethylamine is a secondary amine with two alkyl groups and trimethylamine is a tertiary amine with three surrounding the nitrogen atom.
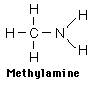
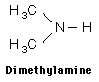
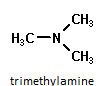
The physical properties of amines depend on the size and number of bonded alkyl groups; however simple amines have specific traits in common. Amines are typically basic compounds due to the lone pair of electrons on the nitrogen which can act as a proton acceptor. They usually have an unpleasant odor of decay, often described as “fishy”. This is not surprising since the amine is found in the basic building blocks of proteins, nucleic acids and chlorophyll in both plants and animals. Two of the amines produced in decaying flesh have names suggestive of their unpleasant smell, cadaverine and putresine. Small, simple amines are usually water soluble with hydrogen bonding occurring between primary and secondary amines. Due to their reactivity, amines are usually physiologically active or toxic. One of the primary uses of amines is in the production of fertilizers as ammonium salts, the acidic form of the amine. These fertilizers are water soluble and readily absorbed by the soil. Crops like rice and sugarcane directly utilize the ammonium form of these fertilizers. Other crops rely on the slow transformation of absorbed ammonium ions to nitrates. The environmental issue is the disruptive effect of the acidity of the residual ammonium salt on the soil which has been found to inhibit native plant growth. It has been documented the acidic soils increase the availability of aluminum, iron, and other metals to be leached from the soil while decreasing the levels of the essential nutrient phosphorous. The Al and Fe when absorbed through the plant roots damages their ability to function and results in weaker plants. Nitrates are compounds containing the polyatomic anion NO3- and are generally found as water soluble salts. Nitrates are mainly produced for use as fertilizers in agriculture because of their high solubility, absorption by plants and biodegradability.
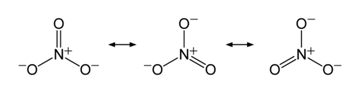
The main nitrates are ammonium, sodium, potassium, and calcium salts, NH4 NO3 , NaNO3, KNO3, and Ca(NO3)2 respectively. The overuse of these highly soluble nitrate fertilizers to maximize crop yields has led to high nitrate levels in runoff into surface water as well as leaching into groundwater. This has been found to be the primary cause of eutrophication, the process where water bodies receive excess nutrients that stimulate excessive plant growth and decay. Nonnative species, those not usually found in the ecosystem, can be favored by this disruption over native plants which will have negative effects on the normal functioning of the ecosystem. An overall reduction in water quality is apparent as cloudy and discolored water fouled by excess decay of plant materials in the water.
Another important family of organic nitrogen containing compounds is the amides, formed by the condensation reaction of a carboxylic acid and an amine.
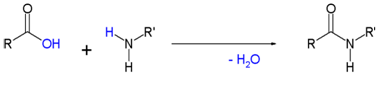
While the general reaction is unfavorable due to the poor leaving group character of the hydroxyl group, chemists typically perform the reaction using heat and compounds which form activated carboxylic acids. Only primary and secondary amines are used in amide formation, while tertiary amines will be unreactive, generating ammonium salts instead.
Because amides are formed by condensation reactions, they have found widespread use in the manufacturing of numerous polymers.
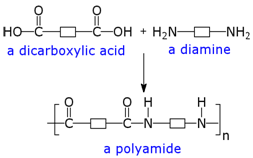
Nylon and Kevlar are both examples of manufactured amide linked polymers.
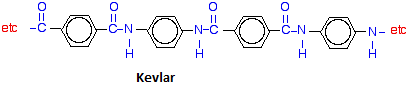
Proteins are biological polymers made by the condensation of amino acids. Each amino acid contains both a carboxylic acid and amine functional group biological and a peptide bond is the result of an amide condensation reaction between two amino acids. For this reason, scientists sometimes refer to the amide backbone of a protein or peptide.
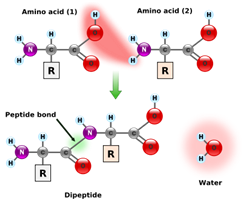
While some amino acids can be synthesized by the human body, others must be obtained from the diet. Of the 22 amino acids required by humans, nine are essential amino acids, which mean that they must be obtained from the diet. Amino acids and proteins are further discussed in the sections on enzymes and in a set of sections devoted to proteins and their chemistry in living systems.
The intermolecular forces and boiling points of nitrogen-containing organic compounds may be explained according to the same principles used for oxygen containing compounds. Since amines can hydrogen bond their boiling points will be higher than similarly sized nitrates or amides. Boiling point will vary according to the degree of alkyl substitution on the central nitrogen atom. Primary amines will form two hydrogen bonds per molecule, secondary amines will form one and tertiary amines will not participate in hydrogen bonding.
Example \(\PageIndex{1}\): Boiling Points
Rationalize the following boiling points: (a) 0°C for CH3CH2CH2CH3; (b) 11°C for CH3CH2OCH3; (c) 97°C for CH3CH2CH2OH; and (d) 170°C for NH2CH2CH2OH.
Solution All four molecules have very similar geometries and the same number of electrons (26 valence electrons plus 8 core electrons), and so their London forces should be about the same.
Compound (a) 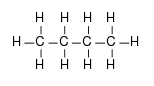 is an alkane and is nonpolar. Its intermolecular forces are the weakest therefore its boiling point will be the lowest.
is an alkane and is nonpolar. Its intermolecular forces are the weakest therefore its boiling point will be the lowest.
By contrast compound (b) 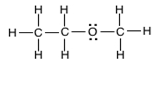 is ether and should be slightly polar. This slight polarity results in a slightly higher boiling point.
is ether and should be slightly polar. This slight polarity results in a slightly higher boiling point.
Compound (c) 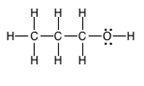 is isomeric with compound (b) but is an alcohol. There is hydrogen bonding between molecules of (c), and its boiling point is much higher since H bonds are the strongest type of intermolecular forces.
is isomeric with compound (b) but is an alcohol. There is hydrogen bonding between molecules of (c), and its boiling point is much higher since H bonds are the strongest type of intermolecular forces.
Molecule (d)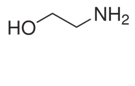 has both an amino group and a hydroxyl group, each of which can participate in hydrogen bonding. Consequently, having two H bonds, it has the greatest amount of intermolecular forces between molecules and the highest boiling point of the four compounds.
has both an amino group and a hydroxyl group, each of which can participate in hydrogen bonding. Consequently, having two H bonds, it has the greatest amount of intermolecular forces between molecules and the highest boiling point of the four compounds.
From ChemPRIME: 8.18: Organic Nitrogen Compounds
Contributors and Attributions
Ed Vitz (Kutztown University), John W. Moore (UW-Madison), Justin Shorb (Hope College), Xavier Prat-Resina (University of Minnesota Rochester), Tim Wendorff, and Adam Hahn.


