Carbon and Fireworks
- Page ID
- 50877
Carbon has thousands, even millions of uses in the world. It also is the essences of who we are as humans. However, one of the most explosive and fun uses of carbon is in fireworks. Fireworks began in the 9th century in China. A Chinese monk by the name Li Tian is credited with being the first person to create fireworks. Every April 18th is now celebrated in China in his honor. The fireworks were used to ward off evil spirits because of their loud bang. Fireworks were used for celebrations of royalty in China for thousands of years. In the 13th century Marco Polo brought fireworks to Europe on one of his voyages. However, the fireworks were not used in Europe for a while, rather the gunpowder that made up the fireworks was used in cannons and guns. The Italians finally were the first to make fireworks in Europe. Fireworks made their way across Europe as well, made in Germany and even showing up in the writing of Shakespeare. King James I of England even knighted the head of his fireworks show at his coronation. American fireworks are relatively new, but Americans have influenced many aspects of fireworks. Previously fireworks were for royalty, or royal celebrations. In America, the first large firework shows were in 1777 for the first anniversary of the Declaration of Independence. The show was for the people, not royalty. It also gave the patriots hope when they were in the midst of the revolutionary war. This signaled a transition of fireworks being used at many celebrations for all kinds of events, rather than just saved for royalty.

In the 1800's Chemists started to discover more and more elements and incorporated these elements into fireworks. Before the 1800's, fireworks consisted of one big flash and an orange light from the carbon igniting. The discovery of potassium (1807), copper compounds, Barium salts (1808), and many other compounds added color and vibrant effects to the fireworks. Below in Table 1 the various elements and their affects on fireworks are shown.
| Symbol | Name | Fireworks Usage |
|---|---|---|
| Al | Aluminum | Aluminum is used to produce silver and white flames and sparks. It is a common component of sparklers. |
| Ba | Barium | Barium is used to create green colors in fireworks, and it can also help stabilize other volatile elements. |
| C | Carbon | Carbon is one of the main components of black powder, which is used as a propellent in fireworks. Carbon provides the fuel for a firework. Common forms include carbon black, sugar, or starch. |
| Ca | Calcium | Calcium is used to deepen firework colors. Calcium salts produce orange fireworks. |
| Cl | Chlorine | Chlorine is an important component of many oxidizers in fireworks. Several of the metal salts that produce colors contain chlorine. |
| Cs | Cesium | Cesium compounds produce indigo color in fireworks. |
| Cu | Copper | Copper compounds produce blue colors in fireworks. |
| Fe | Iron | Iron is used to produce sparks. The heat of the metal determines the color of the sparks. |
| K | Potassium | Potassium compounds help to oxidize firework mixtures. Potassium nitrate, potassium chlorate, and potassium perchlorate are all important oxidizers. The potassium content can impart a violet color to the sparks. |
| Li | Lithium | Lithium is a metal that is used to impart a red color to fireworks. Lithium carbonate, in particular, is a common colorant. |
| Mg | Magnesium | Magnesium burns a very bright white, so it is used to add white sparks or improve the overall brilliance of a firework. |
| Na | Sodium | Sodium imparts a gold or yellow color to fireworks, however, the color is often so bright that it frequently masks other, less intense colors. |
| O | Oxygen | Fireworks include oxidizers, which are substances that produce oxygen in order for burning to occur. The oxidizers are usually nitrates, chlorates, or perchlorates. Sometimes the same substance is used to provide oxygen and color. |
| P | Phosphorus | Phosphorus burns spontaneously in air and is also responsible for some glow in the dark effects. It may be a component of a firework's fuel. |
| S | Sulfur | Sulfur is a component of black powder, and as such, it is found in a firework's propellant/fuel. |
| Sb | Antimony | Antimony is used to create firework glitter effects. |
| Sr | Strontium | Strontium salts impart a red color to fireworks. Strontium compounds are also important for stabilizing fireworks mixtures. |
| Ti | Titanium | Titanium metal can be burned as powder or flakes to produce silver sparks. |
| Zn | Zinc | Zinc is a bluish white metal that is used to create smoke effects for fireworks and other pyrotechnic devices. |
Even though there are many elements within each firework, the main ingredient is still the same: Carbon. Carbon forms the black powder that launches the fireworks into the sky and causes the initial ignition and explosion. Without carbon, there would be no fireworks that got off the ground.
More on Carbon
Carbon forms many known compounds, some of these being diamond which has a three-dimensional network of covalent bonds and graphite which consists of two-dimensional layers covalently bonded. In a diamond structure—each atom is surrounded by four others arranged tetrahedrally. The structure of diamond and graphite is shown below.
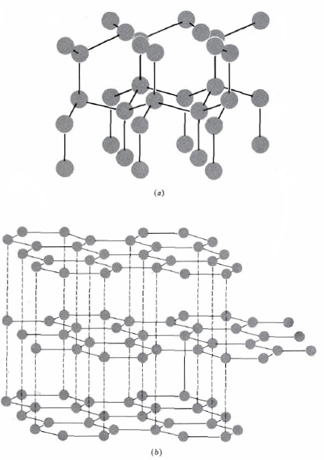
In the table below the ionization energies, especially the third and fourth, are rather large. Formation of true +4 ions is very difficult, and in their +4 oxidation states all group IVA elements form predominantly covalent bonds. The +2 oxidation state, corresponding to use of the np2, but not the ns2, electrons for bonding, occurs for all elements.
| Element | Symbol | Electron Configuration | Usual Oxidation State | Radius/pm | |
|---|---|---|---|---|---|
| Covalent | Ionic (M2+) | ||||
| Carbon | C | [He]2s22p2 | +4, +2 | 77 | - |
| Symbol | Ionization Energy/MJ m ol–1 |
Density/ g cm–3 |
Electro- negativity |
Melting Point (in °C) |
|||
|---|---|---|---|---|---|---|---|
| First | Second | Third | Fourth | ||||
| C | 1.093 | 2.359 | 4.627 | 6.229 | 3.51 | 2.5 | 3550 |
Carbon’s ability to form strong bonds with other carbon atoms and the tremendous variety of organic compounds have already been discussed extensively in the section on organic compounds. You may want to review the subsections dealing with hydrocarbons and the other organic compounds. The most important inorganic carbon compounds are carbon monoxide and carbon dioxide. Both are produced by combustion of any fuel containing carbon:
\[\ce{C + 1/2O2 -> CO}\label{1}\]
\[\ce{CO + 1/2O2 -> CO2}\label{2}\]
The triple bond in 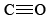 is the strongest chemical bond known, and
is the strongest chemical bond known, and 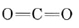 contains two double bonds, and so both molecules are quite stable. Equations \(\ref{1}\) and \(\ref{2}\) occur stepwise when a fuel is burned, and the strong
contains two double bonds, and so both molecules are quite stable. Equations \(\ref{1}\) and \(\ref{2}\) occur stepwise when a fuel is burned, and the strong 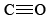 bond makes Eq. \(\ref{2}\) slow unless the temperature is rather high. If there is insufficient O2 or if the products of combustion are cooled rapidly, significant quantities of CO can be produced. This is precisely what happens in an automobile engine, and the exhaust contains between 3 and 4% CO unless pollution controls have been installed.
bond makes Eq. \(\ref{2}\) slow unless the temperature is rather high. If there is insufficient O2 or if the products of combustion are cooled rapidly, significant quantities of CO can be produced. This is precisely what happens in an automobile engine, and the exhaust contains between 3 and 4% CO unless pollution controls have been installed.
CO is about 200 times better than O2 at bonding to hemoglobin, the protein which transports O2 through the bloodstream from the lungs to the tissues. Consequently a small concentration of CO in the air you breathe can inhibit transport of O2 to the brain, causing drowsiness, loss of consciousness, and death. (After a few minutes of breathing undiluted auto exhaust, more than half your hemoglobin will be incapable of transporting O2, and you will faint.) CO in automobile exhaust can be used to put animals to sleep. Because CO is colorless and odorless, your senses cannot detect it, and people must constantly be cautioned not to run cars in garages or other enclosed spaces. With the large number of cars and the great number of miles driven, it is important to limit CO emissions from automobiles. In the early 1970s new EPA standards led to the adoption of catalytic converters, which convert the poisonous CO into CO2. Implementation and increasing effectiveness of these converters has caused CO levels to drop since the 1970s, despite the increase in automobiles on the road.
From ChemPRIME: 12.4: Group IVA
References
- www.fireworks.com/safety/fireworks-history.asp
- en.Wikipedia.org/wiki/Fireworks
- http://www.infoplease.com/spot/fireworks1.html
- library.thinkquest.org/15384/history/index.htm
Contributors and Attributions
Ed Vitz (Kutztown University), John W. Moore (UW-Madison), Justin Shorb (Hope College), Xavier Prat-Resina (University of Minnesota Rochester), Tim Wendorff, and Adam Hahn.


