6: First Chain Reaction (Dec. 2, 1942)
- Page ID
- 19571
Early in World War II the scientific community in the United States, including those Europeans now calling the US their safe home, pursued the idea that uranium fission and the production of excess neutrons could be the source of extraordinary new weapons. They knew Lisa Meitner's interpretation, in Sweden, of Hahn's experiments would likely be known in Germany. Clearly there might now be a race commencing for the development and production of a new, super weapon based on the fission of 235U92 or 239Pu94. By early 1942, it was known that the two naturally occurring isotopes of uranium reacted with neutrons as follows:
- Chain Initiation:
\[\ce{^{235}U_{92}} + \ce{^1n_0} \rightarrow \text{fission products} + (2.5) \ce{^1n_0} + \text{200 MeV Energy} \label{Eq1} \]
- Chain Propagation:
\[ \ce{^{238}U_{92}} + \ce{^1n_0} \rightarrow \ce{^{239}U_{92}} \label{Eq2} \]
\[\ce{^{239}U_{92}} \rightarrow \ce{^{239}Np_{93}} + \beta^{-1} \label{Eq3} \]
with t1/2=23.5 min.
\[\ce{^{239}Np_{93}} \rightarrow \ce{^{239}Pu_{94}} + \beta^{-1} \label{Eq4} \]
with t1/2=2.33 days
Each U-235 that undergoes fission produces an average of 2.5 neutrons (Equation \(\ref{Eq1}\)). In contrast, some U-238 nuclei capture neutrons to become U-239 (Equation \(\ref{Eq2}\)), and subsequently emit two beta particles to produce Pu-239 (Equations \(\ref{Eq3}\) and \(\ref{Eq4}\)). The plutonium was fissile also and would produce energy by the same mechanism as the uranium. A flow sheet for uranium fission is shown below.
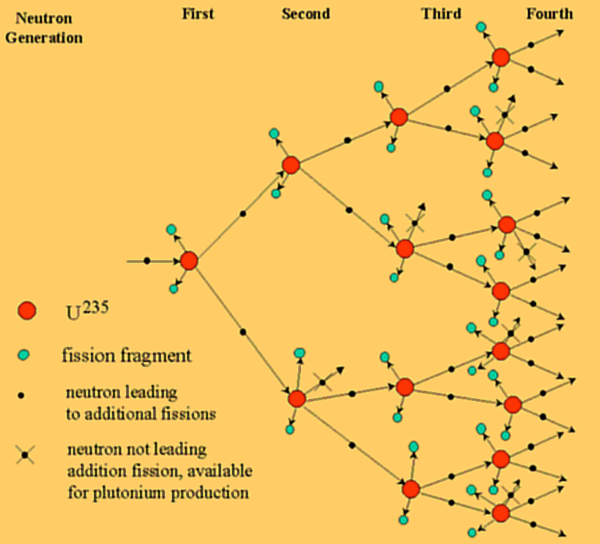
The first generations of a nuclear chain reaction
The answers to two questions were critical to the production of plutonium for atomic bombs:
- Is it possible, using natural uranium (99.3% U-238 and 0.7% U-235), to achieve a controlled chain reaction on a large scale? If so, some of the excess neutrons produced by the fission of U-235 would be absorbed by U-238 and produce fissionable Pu-239.
- How can we separate (in a reasonable period of time) the relatively small quantities of Pu-239 from the unreacted uranium and the highly radioactive fission-product elements.
Although fission had been observed on a small scale in many laboratories, no one had carried out a controlled chain reaction that would provide continuous production of plutonium for isolation. Enrico Fermi thought that he could achieve a controlled chain reaction using natural uranium. He had started this work with Leo Szilard at Columbia University, but moved to the University of Chicago in early 1942.
The first nuclear reactor, called a pile, was a daring and sophisticated experiment that required nearly 50 tons of machined and shaped uranium and uranium oxide pellets along with 385 tons - the equivalent of four railroad coal hoppers - of graphite blocks, machined on site.
The pile itself was assembled in a squash court under the football field at the University of Chicago from the layered graphite blocks and uranium and uranium oxide lumps (Fermi's term) arranged roughly in a sphere with an anticipated 13 foot radius. Neutron absorbing, cadmium coated control rods were inserted in the pile. By slowly withdrawing the rods, neutron activity within the pile was expected to increase and at some point, Fermi predicted, there would be one neutron produced for each neutron absorbed in either producing fission or by the control rods.
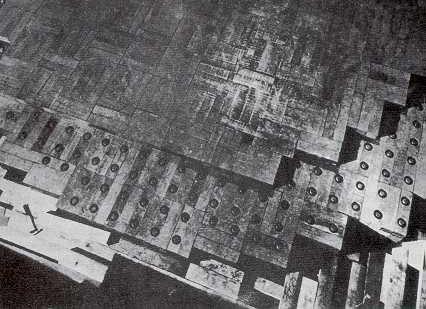
CP-1 - Graphite blocks with 3 inch diameter uranium cylinders inserted - part of a layer of CP-1, the first nuclear reactor. A layer of graphite blocks without inserted uranium is seen covering the active layer.
On December 2, 1942, with 57 of the anticipated 75 layers in place, Fermi began the first controlled nuclear chain reaction occurred. At around 3:20 p.m. the reactor went critical; that is, it produced one neutron for every neutron absorbed by the uranium nuclei. Fermi allowed the reaction to continue for the next 27 minutes before inserting the neutron-absorbing control rods. The energy releasing nuclear chain reaction stopped as Fermi predicted it would.
In addition to excess neutrons and energy, the pile also produced a small amount of Pu-239, the other known fissionable material.
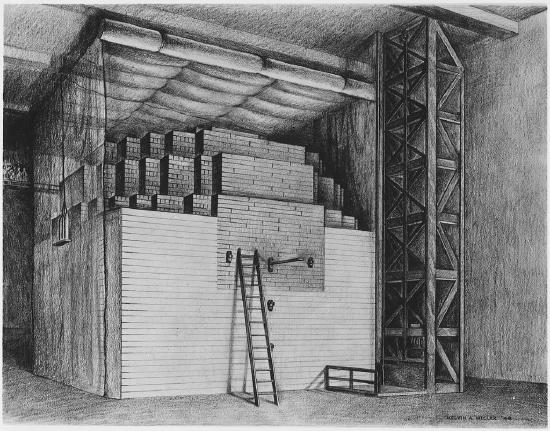
The first controlled chain reaction, Stagg Field, Chicago, Dec. 2, 1942. The first nuclear reactor was erected in 1942 in the West Stands section of Stagg Field at the University of Chicago. On December 2, 1942 a group of scientists achieved the first self-sustaining chain reaction and thereby initiated the controlled release of nuclear energy. The reactor consisted of uranium and uranium oxide lumps spaced in a cubic lattice embedded in graphite. In 1943 it was dismantled and reassembled at the Palos Park unit of the Argonne National Laboratory. (Public Domain; Courtesy of the Argonne National Laboratory)
The achievement of the first sustained nuclear reaction was the beginning of a new age in nuclear physics and the study of the atom. Humankind could now use the tremendous potential energy contained in the nucleus of the atom. However, while a controlled chain reaction was achieved with natural uranium, and could produce plutonium, it would be necessary to separate U-235 from U-238 to build a uranium bomb.
On December 28, 1942, upon reviewing a report from his advisors, President Franklyn Roosevelt recommended building full-scale plants to produce both U-235 and Pu-239. This changed the effort to develop nuclear weapons from experimental work in academic laboratories administered by the U.S. Office of Scientific Research and Development to a huge effort by private industry. This work, supervised by the U.S. Army Corps of Engineers, was codenamed the Manhattan Project. It spread throughout the entire United States, with the facilities for uranium and plutonium production being located at Oak Ridge, Tennessee, and Hanford, Washington, respectively. Work on plutonium production continued at the University of Chicago, at what became known as the Metallurgical Laboratory or Met Lab. A new laboratory at Los Alamos, New Mexico, became the focal point for development of the uranium and plutonium bombs.
Complete Bibliography on Nuclear Physics from the Alsos Digital Library for Nuclear Issues.
Contributors
Frank A. Settle (Washington and Lee University)


