Cisplatin 10. Research as a Career
- Page ID
- 2907
The field of chemistry presents an abundance of career opportunities. Certainly, research propels chemistry (and science in general) forward and gives us a greater understanding of the world around us. The history of cisplatin is an excellent example of the circuitous path scientific research can take in furthering our understanding of the natural world.
American Chemical Society:The society consists of more than 140,000 chemists and other scientists worldwide. It provides meetings, publications and other forums about chemistry by chemists. I made the contacts that led to my preparation of this curriculum unit at a National meeting of the society.
Let's discuss the progress of chemical research that led to the discovery of cisplatin. In a 19th century research laboratory (in 1845, to be exact), Peyrone first synthesized cis-diamminedichloroplatinum(II), also called cis-DDP, and the compound came to be known as Peyrone’s Chloride. It took nearly 50 years before its structure was deduced by Alfred Werner. In a paper published in 1893, Werner discussed both the cis and trans isomers of diamminedichloroplatinum(II). This discussion laid the foundation for coordination chemistry (see module on transition metal chemistry).
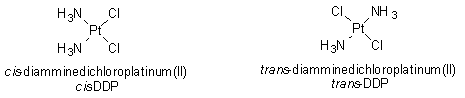
Moreover, knowledge of these compounds was invaluable 70 years later when scientists working in Barnett Rosenberg’s laboratory at Michigan State discovered that the bacterial elongation they were observing was caused by electrolysis products generated from the platinum electrodes they were using—and that the identity of one of these electrolysis products was cis-diamminedichloroplatinum(II). Not only does the discovery of cisplatin show that scientific research can take many unexpected turns, but it also demonstrates that knowledge of many disciplines of science—including chemistry—may be needed to gain a complete understanding of a given problem. Rosenberg, trained as a physicist, needed the expertise of many other scientists to carry out the research program that eventually led to the use of cisplatin as one of the leading chemotherapeutic agents for the treatment of cancer today. When he first wanted to study the effect of electric fields on cell division in E. coli, he hired a microbiologist to work with him. Later, when it became evident that something else was halting bacterial cell division, he added an inorganic chemist who was instrumental in solving the mystery that transition metal complexes were involved. In addition to microbiology and coordination chemistry, the cisplatin project required tapping the expertise of scientists in the fields of molecular biology, biochemistry, biophysics, physiology, pathology, pharmacology, and electron microscopy.1 In fact, Rosenberg commented about his collaborations with medical doctors on this research project in an account he wrote 15 years after the first paper on this topic was published:
I recall two strict admonitions from my major professor when I informed him of my growing interest in biophysics. These were, not to work with medical doctors untrained in research and to avoid cancer research, since many had tarnished their reputations from a malignant neglect of scientific objectivity in their desire to do something useful. I have broken both injunctions, but I cannot say that I am sorry. With a very few exceptions, all connected with the network impressed me as dedicated, selfless, humane scientists. The expected ego clashes and political infighting that characterize so much of science seems to have been muted by the urgency of the problem at hand.1
Interdisciplinary research occurs in many environments. As we are seeing from the cisplatin example, this type of research occurs in academia—in this case, at Michigan State University. This type of research also occurs at pharmaceutical companies such as Merck, where chemists and biologists work together to discover potential drug candidates. In some cases, a natural product is found to have pharmaceutical applications. Often biologists find these natural products, conduct tests on them to see whether they have any useful properties, such as antibacterial, antifungal, or antitumor activity. If the desired activity is found, an analytical chemist then uses various techniques to determine the structure of the compound. At this point, biologists, biochemists, and pharmacologists conduct further studies on the compound to determine how the compound can best be administered (for example, intravenously or orally), how the compound acts in the body, what the appropriate dose levels are, and what potential side effects might occur. While these studies are being conducted, a team of medicinal chemists tries to synthesize the compound in the lab; if the compound may be obtained synthetically—rather than from natural sources only—it can be made more widely available, and it can help more people. Another role medicinal chemists play is to try to make analogs of the compound. Analogs, which are structurally related compounds, are synthesized for several reasons. Sometimes a natural product will cause undesirable or even toxic side effects; an analog may be just as efficacious as the original compound—if not more so—and produce few or no side effects. Furthermore, it may only be possible to administer the original natural product intravenously. Because it is usually more convenient if the drug can be taken orally, an analog may make this possible. (go to the case discussion on the need for funding in pharmaceutical research) In short, interdisciplinary research occurs in many places—including both academia and industry. Finally, chemists are employed in many other areas besides research: business, chemical information, computer-related careers, conservation of art and historic works, consulting, education, entrepreneurial ventures, finance, government work, human resources, law (particularly patent law; see the ChemCase on Gatorade), medicine, professional trade associations, quality assurance and control, regulatory work, science writing, technical sales and marketing, technical service, and technology transfer (see module on profits).2
- Rosenberg, B. In Nucleic Acid-Metal Ion Interactions. T. G. Spiro, Ed. John Wiley & Sons, Inc.: New York, 1980, Vol. 1, pp. 1-29.
- Owens, F. Uhler, R. Marasco, C. Careers for Chemists: A World Outside the Lab. American Chemical Society: Washington, DC, 1997.


