Cisplatin 7. Bioinorganic Chemistry
- Page ID
- 2904
Heavy metals, such as platinum, have long been considered toxic to the human body. The discovery of cisplatin (in which the central atom is platinum) as an effective antitumor agent therefore came as a surprise to the medical community. As it turns out, a great many metals are vitally important in biology. The study of metals in biological systems is called bioinorganic chemistry. Biologically significant metals can be split into two classes: naturally occurring metals in biological systems and metals that are used for probes or drugs in biological systems.1 (See Figure 1.)

An astounding number of metals occur naturally in biological systems and are, in fact, required for biological function. For example, magnesium is found in chlorophyll, which is necessary for photosynthesis; both chlorophyll and iron-containing heme groups are found in the photosynthetic reaction center. Cobalt is found in coenzyme B12, which is essential for the transfer of alkyl groups from one molecule to another in biological systems, as well as the reduction of the ribose ring in ribonucleotides that make up RNA to the deoxyribose ring in deoxyribonucleotides that make up DNA. Nickel is found in factor F430, which is required for methanogenesis, a process used by the archaeobacteria in which the simple gases, such as H2, CO, and CO2, are used to provide both energy and a carbon source. Iron is found in a variety of iron-sulfur clusters, which are necessary for nitrogen fixation, as well as in heme groups, found in hemoglobin, which is used for dioxygen transport and storage in the body.1,2 (See Figure 2.)
 |
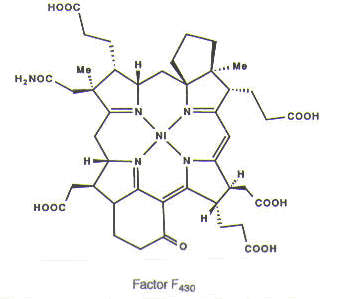 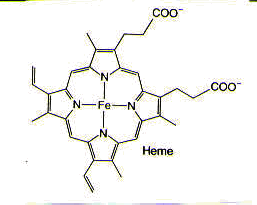 |
Figure 2. Metals in biological systems: magnesium in chlorophyll, which is found in the photosynthetic reaction center; cobalt in coenzyme B12; nickel in factor F430; and iron in heme, which is found in hemoglobin. Reprinted with permission.1,2
Metals have also been introduced into biological systems to probe structure and function of those systems. For example, heavy metals such as mercury and platinum are employed to help determine the structure of macromolecules by x-ray crystallography and electron microscopy. Furthermore, metal-containing compounds are used to diagnose a variety of conditions. For example, a technetium radiopharmaceutical, called cardiolyte, supplies 99mTc, which is selectively taken up by myocardial tissue (the muscle tissue found in the heart) and used to image the heart. Finally, metals are found in drugs used to treat a variety of diseases and ailments. Among them is a gold-containing agent called Auranofin used to treat rheumatoid arthritis; unlike other antiarthritic gold agents, this one can be taken orally. Also, as we have seen, a platinum-containing compound called cisplatin is used as a chemotherapeutic agent in the treatment of testicular, ovarian, and head and neck tumors.1(See Figure 3.)
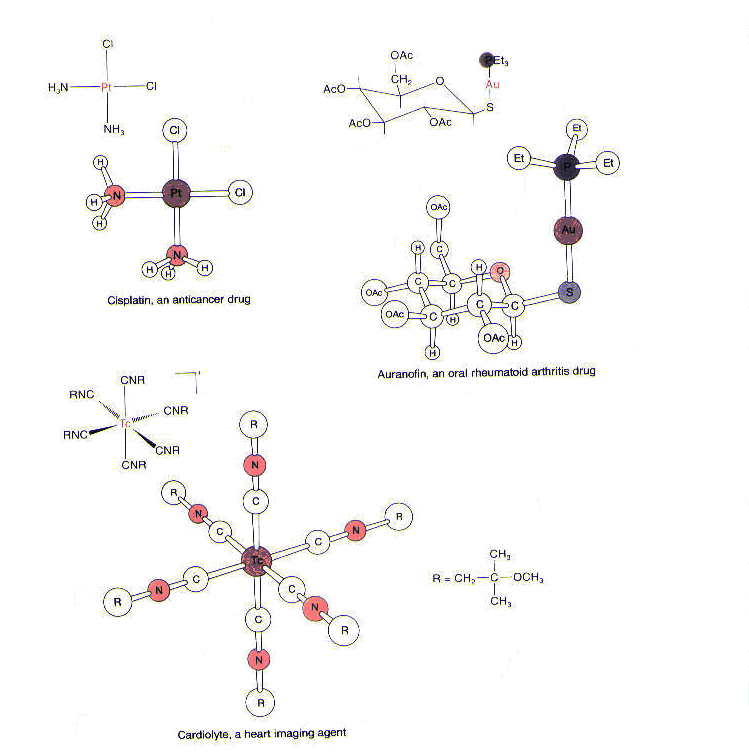
In short, many metals play an extremely important role in biology. Some metals are essential for biological function and are found in enzymes and cofactors required for various processes. In addition, some metals have been used to explore biological systems; still others have been used to treat a variety of diseases and conditions.
References
- Lippard, S. J., Berg, J. M. Principles of Bioinorganic Chemistry. University Science Books: Mill Valley, CA, 1994.
- Crabtree, R. H. The Organometallic Chemistry of the Transition Metals. John Wiley & Sons, Inc.: New York, 1988.


