Cisplatin 5. The Role of Platinum Electrodes
- Page ID
- 2902
As we saw in the module on control experiments, Barnett Rosenberg and his coworkers wanted to find out if any oxidizing agents were generated in the nutrient medium used for growing E. coli bacterial cells following electrolysis. They isolated various components of the medium, subjected them to electrolysis, and then tested them for the presence of an oxidizing agent with the potassium iodide-starch test (described in the control experiments module).
The researchers found that many solutions containing either ammonium ion or chloride ion or both gave a positive test. Specifically, the following ionic compounds present in the nutrient medium formed oxidizing agents after electrolysis with platinum electrodes: ammonium sulfate ((NH4)2SO4); ammonium carbonate ((NH4)2CO3); ammonium chloride (NH4Cl); and other chlorides. Sodium chloride (NaCl) gave a faint positive response.1 The researchers had finally found an oxidizing agent formed during electrolysis, and they believed that this new compound (or compounds) might be responsible for inhibiting cell division in E. coli bacteria. Their quest now was to determine the identity of this new compound. They knew that platinum electrodes could be attacked by an acidified chloride solution during electrolysis, generating platinum hexachloride, PtCl62-, and they hypothesized that a soluble platinum salt was the reactive compound. That platinum, a supposedly chemically inert material, might actually be reacting to form the causative agent in inhibiting bacterial cell division was an unexpected result indeed. To test the notion that a soluble platinum salt might be responsible for halting mitosis in E. coli, an inorganic chemist in Rosenberg’s group made up a solution of the ammonium salt of platinum hexachloride ((NH4)2PtCl6), in which platinum existed in its +4 oxidation state. The positively charged ammonium ions acted as counterions to the negatively charged platinum hexachloride ion.
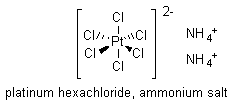
When (NH4)2PtCl6 was subjected to the potassium iodide-starch test, the results were identical to those obtained when the nutrient medium was electrolyzed, as long as there was a sufficient concentration of platinum present (>100 p.p.m.)—meaning that (NH4)2PtCl6 could be an oxidizing agent. This new result suggested that a soluble platinum salt, generated from the platinum electrodes during electrolysis, in combination with the ions present in the nutrient medium, might be the active agent in inhibiting cell division and leading to filamentous growth. Indeed, when this soluble platinum salt ((NH4)2PtCl6) was used to inoculate the culture chamber used in the original experiments (see the electric fields module), the E. coli bacterial cells formed filaments!1
[Note: A later account suggests that (NH4)2PtCl6 at first caused cell death—then later, after standing on a laboratory shelf for a few weeks, produced a small number of filaments. This will be discussed more in the discovery of cisplatin module.]
The researchers did not stop there: they wanted to know whether other transition metals in group VIIIB, meaning those in groups 8, 9, and 10 (see the transition metal chemistry module), would have a similar effect on E. coli bacterial cells. For this reason, they made and tested many more compounds containing various transition metals, including cobalt (Co), iridium (Ir), nickel (Ni), osmium (Os), palladium (Pd), rhodium (Rh), and ruthenium (Ru), as well as several other platinum-containing compounds. As it turned out, several of the transition metal compounds tested were toxic to bacterial cells; these are listed in column A of Table 1. Another group of compounds caused no change to the bacterial cells; these are listed in column B. Finally, several transition metal compounds caused elongation of the bacterial cells, just as (NH4)2PtCl6 did. Many of these compounds contained platinum, but others contained rhodium or ruthenium.
Table 1. Effects of group VIIIB transition metal compounds on bacterial growth. (Concentrations of metal ions maintained for 2 h at 8 p.p.m. in the continuous culture chamber.)1
|
A. Caused bacterial death |
B. Caused no change |
C. Caused elongation |
|
CoCl2 |
[Co(NH3)6]Cl2 |
K+,NH4+,H+—[PtCl6]2- |
|
(NH4)2IrCl6 |
K2Ir(NO2)6 |
(NH4)2PtBr6 |
|
NiCl2 |
[Ni(NH3)6]Cl2 |
(NH4)2PtI6 |
|
(NH4)2OsCl6 |
[Pt(en)3]Cl4 |
|
|
(NH4)2PdCl4 |
cis [Rh(en)2Cl2]NO3 |
RhCl3 |
|
[Rh(NH3)5Cl]Cl2 |
trans[Rh(en)2Cl2]NO3 |
(NH4)3RhCl6 |
|
PdCl2 |
[Ru(NH3)4ClOH]Cl |
Although the researchers found several compounds that inhibited cell division of E. coli without interfering with cell growth, they were not yet able to define the roles played by either the metal oxidation states or the ligands. Having found the answers to many of the questions that came up during their study of the effect of electromagnetic fields on cell division in E. coli, Rosenberg and his coworkers found that many new questions arose. Indeed, a well-designed experiment often leads to answers as well as new questions. They posed some of these questions at the end of their first publication on this topic:
- "What is the mechanism of action of these metal ions?
- Where is the locus of action in the bacterial cell?
- How does the effect of these metal ions relate to the actions of the other causative agents of filamentous growth—is there a weak link that all operate on?
- Can these metal ions inhibit cell division in other bacteria, or cells?"1
We will see that subsequent studies, conducted by both Rosenberg’s group and by other research groups, provided answers to these new questions.
References
- Rosenberg, B., Van Camp, L., Krigas, T. Nature , 1965, 205, pp. 698-699.


