Spontaneous Combustion Reaction of Acetylene with Chlorine
- Page ID
- 3042
Chemical Concepts Demonstrated
- Exothermicity
- Spontaneous combustion
- Chemistry and reactivity of chlorine and hypochlorite ion
- Carbide chemistry
- Synthesis of acetylene
Demonstration
| The beaker contains HCl and CaC2 pellets and is covered.
Sodium hypochlorite (Chlorox) is added to the beaker. |
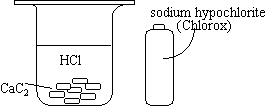 |
Observations
Flames shoot out from the top of the beaker.
Explanation (including important chemical equations)
Calcium carbide reacts with water to form acetylene.
CaC2 (s) + 2 H2O (l) --> H2C2 (g) + Ca(OH)2 (aq)
Chlorine gas is generated when hypochlorite solutions are acidified. This is the opposite of the disproportionation that chlorine gas undergoes under basic conditions, so the reaction has been referred to as a conproportionation.
OCl- (aq) + HCl (aq) --> Cl2 (g) + H2O (l)
The chlorine gas reacts with the acetylene (as well as many other hydrogenated compounds). This reaction is not what one might first expect, since it is not the product of the addition of the halogen across the carbon-carbon triple bond. The product is formed by the abstraction of hydrogen from the hydrocarbon.
H2C2 (g) + Cl2 (g) --> HCl (aq) + HC2Cl (g)

