A Solid-Vapor Equilibrium Demonstration
- Page ID
- 2968
Chemical Concept Demonstrated
- Temperature's effect on the equilibrium between a solid and its vapor
Demonstration
The two round-bottom flasks contain both iodine and iodine vapor.
Compare the intensity of the color in the flasks and their temperatures. |
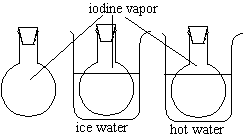 |
Observations
There appears to be more purple vapor in the heated flask.
Explanation
Heating the flask increases the vapor pressure of the solid iodine inside of it. When the vapor presure increases, the equilibrium between the solid and the vapor shifts to favor the vapor. Therefore, the heated flask has more of the purple iodine vapor floating about the inside of it.
It is possible to place a flask of iodine and iodine vapor in an ice water bath, but because the flask at room temperature has very little vapor to begin with, it is unlikely that an audience will see a significant difference between the room temperature flask and the ice water flask.
Contributors
- Dr. George Bodner (Perdue University)

