The Catalytic Decomposition of Hydrogen Peroxide
- Page ID
- 3055
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\)
Chemical Concepts Demonstrated
- Chemisty of hydrogen peroxide
- Disproportation reactions
- Enzyme catalysis
Demonstration
|
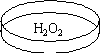 |
Observations
The various items foam in the dishes.
Explanation (including important chemical equations)
Hydrogen peroxide undergoes disproportionation. Both oxidation and reduction occur at the same time.
2 H2O2 (aq) ---> 2 H2O (l) + O2 (g) Enthalpy: -196.1 kJ/mol
The activation energy of the reaction is about 75 kJ/mol in the absence of catalyst. Platinum metal catalysts can lower the activation energy to about 49 kJ/mol. The catalase enzyme (found in blood) lowers the activation energy to below 8 kJ/mol, which corresponds to an increase in the rate of reaction at physiologial temperatures by a factor of 2 x 10 11 or more.
Contributors
- Dr. George Bodner (Perdue University)

