Volume of Mixing Demonstration
- Page ID
- 3100
Chemical Concepts Demonstrated
- Spaces between particles in a solution
- Liquid lattices
- Polarity
Demonstration
| 500 mL of 190-or 200-proof ethanol is added to 500 mL of water. | 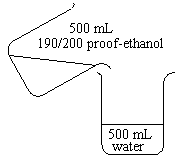 |
Observations
The two liquids combine to form about 970 ml of solution.
Explanation
Both of these liquids are polar. As such, they create intermolecular lattices, arranging themselves on the basis of the charges they carry and hydrogen bonding. Because of the necessary shapes of these lattices, these liquids don't "pack" (i.e. form lattices that conserve space) as well as if they were nonpolar. This creates spaces between the molecules.
When the two liquids are mixed, the molecules of the liquids can go into the other liquid's available spaces. They can do this because they are both polar and can mix freely. Because the molecules of the two liquids are different sizes, their resulting intermolecular lattice can be better arranged to conserve more space. The mixture of the two liquids takes up less volume than the two liquids did separately.

